Abstract
ACKNOWLEDGMENTS
REFERENCES
Fig. 1.
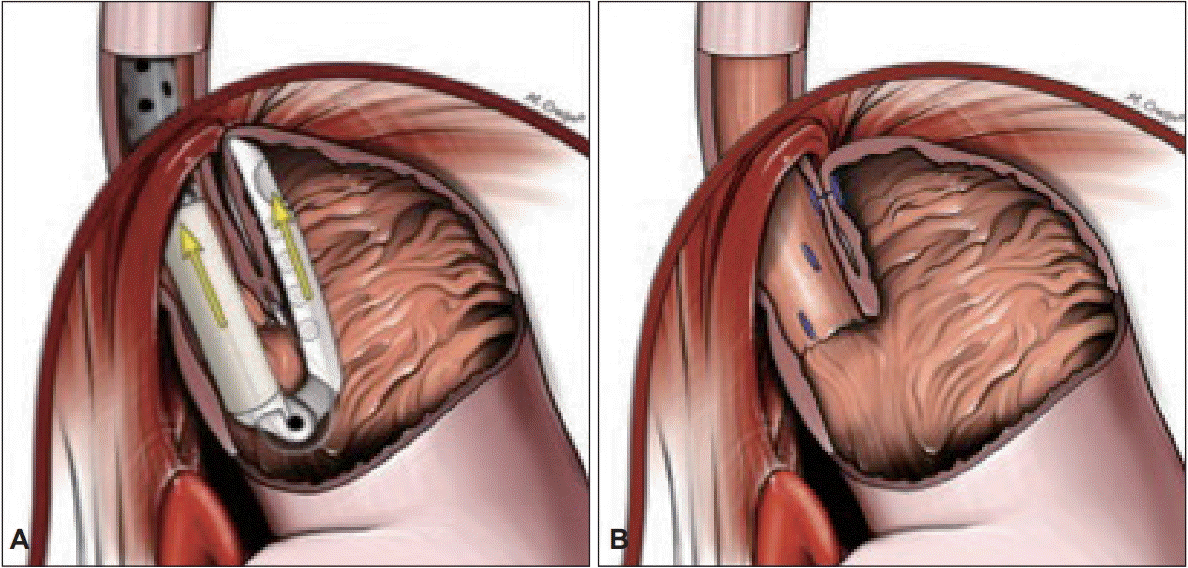
Table 1.
| Study/country | Type of study | Intervention | Indication | Exclusion criteria | Total subjects/subjects lost to follow-up | Instrument | Technique/extent of wrap around the esophagus/length of reconstructed valve | Clinical response | Endoscopic response | PPI requirement | Follow-up period | Peri-procedure complications (requiring extended hospital stay) | TIF failure requiring second endoscopic/surgical intervention | Other complications (persistent >1 mo, post TIF) | Miscellaneous facts |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trad et al. (2014) [8] USA | Prospective, randomized, multicenter, crossover study | TIF |
1. Daily regurgitation or atypical symptoms (Montreal criteria) on PPI 2. Abnormal 48 hr ambulatory pH test 3. H/O daily PPI use for at least 6 mo |
1. BMI >35 2. Barrett’s esophagus >2 cm 3. Hill grade valve III or IV 4. Hiatal hernia >2 cm in either dimension 5. Los Angeles grade C or D classification |
40/1 | EsophyX-2 device via flexible endoscope | TIF 2.0 protocol |
1. Resolution of regurgitation and atypical symptoms 1) 6 mo: 13/20 (65%) 2) 12 mo: 30/39 (77%) 2. Mean GERDHRQL 1) Baseline: 26.25 2) 6 mo: 5.23 3) 12 mo: 5.41 3. Mean RDQ 1) Baseline: 2.91 2) 6 mo: 0.35 3) 12 mo: 0.50 4. Mean RSI 1) Baseline: 22.00 2) 6 mo: 4.64 3) 12 mo: 4.79 |
1. Healed esophagitis 1) 6 mo: 18/20 (90%) 2) 12 mo: 19/19 (100%) 2. Normalized esophageal pH 1) 6 mo: 21/39 (54%) 2) 12 mo: 17/38 (45%) 3. Mean Demeester score (48 hr pH study) 1) Baseline: 35.28 2) 6 mo: 23.64 3) 12 mo: 25.32 |
1. Baseline 1) Daily: 100% 2) Occasional: 0% 3) None: 0% 2. 6 mo 1) Daily: 2% 2) Occasionally: 8% 3) None: 90% 3. 12 mo 1) Daily: 3% 2) Occasionally: 15% 3) None: 82% |
12 mo | None | None | Flatulence: 1/39 | - |
| High dose PPI for first 6 mo, followed by TIF | 23/2 | Maximal labelled dose of PPI, split into twice daily regimen for 6 mo followed by EsophyX-2 device via flexible endoscope | TIF 2.0 protocol |
1. Resolution of regurgitation and atypical symptoms 1) 6 mo: 1/21 (0.05%) 2) 12 mo: 6/9 (67%) 2. Mean GERDHRQL 1) Baseline: 26.43 2) 6 mo: 18.86 3) 12 mo: 10.05 3. Mean RDQ 1) Baseline: 3.04 2) 6 mo: 2.14 3) 12 mo: 1.33 4. Mean RSI 1) Baseline: 22.62 2) 6 mo: 19.62 3) 12 mo: 8.76 |
1. Healed esophagitis 1) 6 mo: 5/13 (38%) 2) 12 mo: 11/13 (85%) 2. Normalized esophageal pH 1) 6 mo: 11/21 (52%) 2) 12 mo: 7/21 (33%) 3. Mean Demeester score (48 hr pH study) 1) Baseline: 35.79 2) 6 mo: 19.29 3) 12 mo: 28.60 |
1. Baseline 1) Daily: 100% 2) Occasionally: 0% 3) None: 0% 2. 6 mo 1) Daily: 100% 2) Occasionally: 0% 3) None: 0% 3. 12 mo 1) Daily: 10% 2) Occasionally: 9% 3) None: 71% |
None | None | None | ||||||
| Toomey et al. (2014) [9] USA | Case-control study with prospective follow-up | TIF | GERD refractory to or requiring open ended medical therapy |
1. Hiatal hernia >2 cm 2. Esophageal dysmotility |
20 | EsophyX-2 device via flexible endoscope | DNA |
1. Patient satisfaction at follow-up: 67% 2. Patients with symptom frequency (<1/mo): 83% |
DNA | DNA | DNA | None | None | None | OT: 71 min HSL: 1 day |
| Toupet fundoplication | GERD refractory to or requiring open ended medical therapy with abnormal esophageal motility | 1. Failed surgical fundoplication in past | 20 | DNA | LESS |
1. Patient satisfaction at follow-up: 92% 2. Patients with symptom frequency (<1/mo): 92% |
DNA | DNA | DNA | DNA | NA | DNA | OT: 85 min HSL: 1 day 10%: operation related complication (severity not reported) | ||
| Nissen fundoplication | GERD refractory to or requiring open ended medical therapy with normal esophageal motility |
1. Failed surgical fundoplication in past 2. Esophageal dysmotility |
20 | DNA | LESS |
1. Patient satisfaction at follow-up: 86% 2. Patients with symptom frequency (<1/mo): 80% |
DNA | DNA | DNA | DNA | NA | DNA | OT: 119 min HSL: 2 day 5%: procedure related complication (severity not reported) | ||
| Wilson et al. (2014) [10] USA | Prospective multicenter trial | TIF | Chronic GERD (>1 yr), daily PPI use >6 mo, with unsatisfactory response |
1. Hiatal hernia >2 cm (axial), >3 cm (transverse) 2. BMI >35 3. Esophageal achalasia 4. Barretts esophagus >2 cm 5. Reflux esophagitisgrade D Los Angeles classification 6. Miscellaneous: gastroparesis, Zenker diverticulum, gastroparesis, scleroderma |
100/4 | EsophyX-2 device via flexible endoscope | TIF 2.0 protocol 240–330 2–5 cm |
1. Median GERDHRQL 1) Baseline: 24 2) 6 mo: 4 3) 12 mo: 2 2. Median GERSS 1) Baseline: 26 2) 6 mo: 4 3) 12 mo: 4 3. Median RSI 1) Baseline: 20 2) 6 mo: 5 3) 12 mo: 4 |
1. Esophagitis 1) Healed (12 mo): 13/17 (76%) 2) Improved (12 mo): 2/17 (12%) 2. Esophageal acid exposure 1) Normalization (12 mo): 14/27 (52%) |
1. Baseline 1) Daily: 92% 2) Occasionally: 8% 3) None: 0% 2. 6 mo 1) Daily: 11% 2) Occasionall: 9% 3) None: 80% 3. 12 mo 1) Daily: 23% 2) Occasionally: 3% 3) None: 74% |
12 mo | None |
1. 5/96: underwent LNF (1 had severe vomiting post procedure, 2 were non compliant with dietary instructions, other 2-unspecified reason) 2. 1/96: underwent repeat TIF |
At 12 mo 1) De novo dysphagia: 2 2) De novo bloating: 1 3) Worsening flatulence: 2 |
- |
| Cadière et al. (2008) [11] | Prospective multicenter | TIF | 18.80 yr, chronic GERD (>6 mo) responsive to PPI therapy, with symptom recurrence on discontinuation of PPI for 14 day |
1. Irreducible hiatal hernia >2 cm 2. Previous failed anti-reflux surgery 3. BMI ≥35 4. Delayed gastric emptying 5. Esophageal diseasemotility disorder, ulcer, biopsy proven Barretts, stricture 6. Reflux esophagitisgrade D Los Angeles classification |
86/7 | EsophyX-2 device via flexible endoscope | TIF 1.0 protocol 230 (160–300) 4 cm (2–6) |
Median GERDHRQL 1) Baseline: 24 2) 6 mo: 5 3) 12 mo: 7 |
Median Demeester score 1) Baseline: 34 2) 6 mo: 24 3) 12 mo: 28 |
1. Baseline 1) Daily: 100% 2) Occasional: 0% 3) None: 0% 2. 6 mo 1) Daily: 14/86 (17%) 2) Occasionally: 14% 3) None: 69% 3. 12 mo 1) Daily: 12/86 (15%) 2) Occasionally: 16% 3) None: 68% |
12 mo |
1. Esophageal perforation 2/86: successfully repaired surgically 2. Post TIF intraluminal bleedingtreated with endoscopic clips, fibrin glue, and blood transfusion |
3/86 LNF (2 were post perforation during TIF, and the third one-reason not specified) |
1. Abdominal pain: 1/86 2. Nausea: 1/86 |
|
| Rinsma et al. (2015) [12] Netherlands | Prospective, randomized, controlled, multicenter trial | Continuation of PPI therapy | Chronic GERD (>6 mo), partially responsive to acid suppressive medication |
1. Hiatal hernia >2 cm 2. Previous failed anti-reflux surgery 3. BMI ≥35 4. Esophageal motility disorder on manometry 5. Barretts esophagus 6. Reflux esophagitisgrade D Los Angeles classification |
15 | NA | NA |
Mean GERD-HRQL 1) Baseline: 26.0 2) 6 mo: 23.6 |
1. Distal baseline impedance (Ω) 1) Baseline: 1,088 2) 6 mo: 2,470 2. Acid exposure time (%) 1) Baseline: 12.4 2) 6 mo: 5.9 3. Mean acid reflux episodes 1) Baseline: 65.6 2) 6 mo: 33.9 |
NA | 6 mo | NA | NA | NA | - |
| TIF | 32 | EsophyX-2 device via flexible endoscope | DNA |
Mean GERD-HRQL 1) Baseline: 23.7 2) 6 mo: 8.5 |
1. Distal baseline impedance (Ω) 1) Baseline: 1,769 2) 6 mo: 2,294 2. Acid exposure time (%) 1) Baseline: 9.7 2) 6 mo: 6.9 3. Mean acid reflux episodes 1) Baseline: 63.2 2) 6 mo: 39.3 |
DNA | 6 mo | DNA | None | DNA | |||||
| Rinsma et al. (2014) [13] Netherlands | Prospective single center study | TIF | Chronic GERD (>6 mo), on PPI therapy, dissatisfied or unwilling to continue lifelong PPI therapy |
1. Hiatal hernia >2 cm in length 2. Previous failed anti-reflux surgery 3. Esophageal stricture or ulcer 4. Esophageal motility disorder on manometry 5. Barretts esophagus 6. Reflux esophagitisgrade D Los Angeles classification 7. Current pregnancy 8. Severe comorbidity-cardiopulmonary disorder, coagulation disorder, portal hypertension, immunosuppression, morbid obesity |
15 | EsophyX-TM device via flexible endoscope | TIF 2.0 protocol |
Mean GERD-HRQL 1) Baseline: 27.5 2) 6 mo: 13.2 |
1. Mean EGJ distensibility (mm2/mm of Hg) 1) Baseline: 2.4 2) 6 mo: 1.6 2. 24-hr ambulatory impedance pH (upright acid exposure time) (%) 1) Baseline: 11.7 2) 6 mo: 6.6 3. 24-hr ambulatory impedance pH (liquid reflux episodes) (%) 1) Baseline: 30.4 2) 6 mo: 16.7 4. Total no. of TLESR 1) Baseline: 16.8 2) 6 mo: 9.2 |
1. Baseline 1) Daily: 100% 2) None: 0% 2. 6 mo 1) Daily (same dose): 1/15 (6.7%) 2) Daily (decreased dose): 3/15 (20%) 3) None: 11/15 (73.3%), but 1 used non PPI antisecretory medication |
6 mo | DNA | None | DNA | - |
| Testoni et al. (2015) [14] Italy | Prospective single center study | TIF | Symptomatic GERD, on PPI (standard dose twice a day) for a minimum of 3 mo |
1. Hiatal hernia >3 cm 2. Previous esophageal, gastric or major abdominal surgery 3. Atypical GERD symptoms 4. Biopsy prove Barretts esophagus 5. Esophageal stricture 6. Severe comorbidity-cardiopulmonary disease and collagen disease |
50 | EsophyX-TM device via flexible endoscope | TIF 2.0 protocol |
1. Mean GERDHRLQ 1) Baseline (on PPI): 20 2) Baseline (off PPI): 46 3) 12 mo: 16 4) 24 mo: 17 2. Mean GERDQUAL 1) Baseline (on PPI): 84 2) Baseline (off PPI): 114 3) 12 mo: 71 4) 24 mo: 80 |
1. pH metry, Johnson Demeester score 1) Baseline: 22 2) 6 mo: 18 3) 24 mo: 19 2. Impedance, total refluxes (number) 1) Baseline: 66 2) 6 mo: 38 3) 24 mo: 43 3. LES pressure (mm Hg) 1) Baseline: 8 2) 6 mo: 11 3) 24 mo: 12 |
1. 6 mo 1) Stopped PPI: 61.2% 2) Halved PPI: 22.5% 3) On PPI: 16.3% 2. 12 mo 1) Stopped PPI: 51.0% 2) Halved PPI: 28.6% 3) On PPI: 20.4% 3. 6 yr 1) Stopped PPI: 35.7% 2) Halved PPI: 50.0% 3) On PPI: 14.3% |
6 yr | Pneumothorax: 2 subjectstreated successfully with immediate transthoracic drainage | 4/50 LNF at 12 mo secondary to persistent GERD symptoms | DNA | - |
| Trad et al. (2012) [15] USA | Retrospective | TIF |
1. Persistent GERD and/LPR symptoms, not/partial controlled on antisecreatory medications 2. Either dissatisfied with current therapy or unwilling to continue taking medications indefinitely |
1. Hiatal hernia, axial dimension >2 cm | 34/6 | EsophyX-2 device via flexible endoscope | TIF 2.0 protocol 270 (240–300) 3 cm (2–4) |
1. Median GERDHRQL 1) Baseline: 26 2) 14 mo: 4 2. Median GRESS score 1) Baseline: 24 2) 14 mo: 3 3. Median RSI 1) Baseline: 17 2) 14 mo: 4 |
24-hr pH (only 2 subjects) 1. First subject: Demeester score 1) Baseline: 29 2) Post-TIF at 14 mo: 24.5 2. Second subject 1) Baseline: abnormal pH 2) Post-TIF at 14 mo: normal pH |
1. Baseline 1) Daily: 25/28 (89%) 2) Occasional: 3/28 (11%) 3) None: 0/28 2. 14 mo 1) Daily: 5/28 (18%) 2) Occasionally: 5/28 (18%) 3) None: 18/28 (64%) |
14 mo | None | 1/28 LNF (likely cause failure of dietary restriction post-TIF, causing disruption of reconstructed valve) | None | - |
| Kumta et al. (2015) [16] USA | Case report | TIF | Subject with achalasia, who underwent peroral endoscopic myotomy and developed reflux symptoms refractory to PPI | None | 1 | DNA | 270 | DNA | DNA | DNA | DNA | DNA | DNA | DNA | - |
| Hunter et al. (2015) [17] USA Prospective sham controlled mutlicenter study | TIF f/b 6 mo of placebo treatmen | 18.80 yr age group with chronic GERD (>6 mo) symptoms and troublesome regurgitation despite daily PPI (40 mg) use |
1. Systemic disease: not well controlled 2. BMI >35 3. Esophageal ulcer or stricture 4. Current pregnancy or plan of pregnancy in next 12 mo 5. Hiatal hernia >2 cm 6. Barrett’s esophagus >2 cm 7. Esophagitis: Grade C or D Los Angeles classification 8. Esophageal dysmotility 9. Previous esophageal or gastric surgery 10. Peptic ulcer disease 11. Gastric outlet obstruction or gastroparesis 12. Portal hypertension or coagulopathy 13. Immunosuppression |
81/1 | EsophyX-2 device via flexible endoscope | TIF 2.0 protocol 270 (200–340) 3 cm (midportion); 1 cm (either corner) |
1. Elimination of troublesome regurgitation (6 mo): 54/81 (67%) 2. Median regurgitation RDQ score 1) Baseline (on PPI): 3.5 (3.4.3) 2) 6 mo (on placebo): 0.5 (0.1.5) 3. Median Heartburn RDQ score 1) Baseline (on PPI): 2.6 (1.5.3.8) 2) 6 mo (on placebo): 0.5 (0.1.6) 4. Composite median heartburn and regurgitation RDQ score 1) Baseline (on PPI): 3.1 (2.4.3.8) 2) 6 mo (on placebo): 0.6 (0.1.3) |
1. Total no. of reflux episodes 1) Baseline: 135 2) 6 mo: 94 2. Percent time pH <4 1) Baseline: 9.3 2) 6 mo: 6.4 3. DeMeester Score 1) Baseline: 33.6 2) 6 mo: 23.9 |
By ITT analysis 1) 3 mo: 10/87 (11%) resumed PPI 2) 18 mo: 24/87 (28%) resumed PPI |
6 mo | None |
By ITT analysis 1. Treatment failure 1) 3 mo: 10/87 (11%), all resumed PPI, no other intervention done 2) 18 mo: 24/87 (28%), all resumed PPI, no other intervention done |
None | - | |
| Sham surgery f/b 6 mo of PPI therapy | 38/1 | EGD and 15 F maloney dilator | 30 min for EGD and 15 min for Maloney dilator |
1. Elimination of troublesome regurgitation (6 m): 17/38 (45%) 2. Median Regurgitation RDQ score 1) Baseline (on PPI): 3.8 (2.9.4.5) 2) 6 mo (on PPI): 0.8 (0.2) 3. Median Heartburn RDQ score 1) Baseline (on PPI): 3.0 (2.0.4.1) 2) 6 mo (on PPI): 0.8 (0.2) 4. Composite heartburn and regurgitation RDQ score 1) Baseline (on PPI): 3.3 (2.5.4.0) 2) 6 mo (on PPI): 0.9 (0.1.2.0) |
1. Total no. of reflux episodes 1) Baseline: 125 2) 6 mo: 122 2. Percent time pH <4 1) Baseline: 8.6 2) 6 mo: 8.9 3. DeMeester score 1) Baseline: 30.9 2) 6 mo: 32.7 |
DNA | 6 mo | None |
By ITT analysis 1. Treatment failure 1) 3 mo: 15/42 (35.7%), 12/15 underwent crossover to TIF 2) 18 mo: 30/42 (71.4%) underwent crossover to TIF |
NA |
PPI, proton pump inhibitor; TIF, transoral incisionless fundoplication; H/O, history of; BMI, body mass index; GERD-HRQL, gastroesophageal reflux disease-health-related quality of life; RDQ, reflux disease questionnaire; RSI, reflux symptom index; DNA, data not available; LESS, laparo-endoscopic single site; NA, not available; OT, operating time; HSL, hospital stay length; GERSS, GERD symptom score; LNF, laparoscopic Nissen fundoplication; EGJ, esophagogastric junction; TLESR, transient lower esophageal sphincter relaxation; LPR, laryngopharangeal reflux; f/b, followed by; ITT, intention-to-treat analysis.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download