Abstract
Advances in technology have facilitated the common use of small-bowel imaging. Intraoperative enteroscopy was the gold standard method for small-bowel imaging. However, noninvasive capsule endoscopy and invasive balloon enteroscopy are currently the main endoscopic procedures that are routinely used for small-bowel pathologies, and the indications for both techniques are similar. Although obstruction is a contraindication for capsule endoscopy, it is not considered to be problematic for double-balloon enteroscopy. The most important advantage of double-balloon enteroscopy is the applicability of therapeutic interventions during the procedure; however, double-balloon enteroscopy has certain advantages as well as disadvantages.
Push and sonde enteroscopy were widely used for small-bowel endoscopy prior to the development of double-balloon enteroscopy (DBE). However, due to the insufficient depth of intubation into the small bowel and the high rate of complications with push and sonde enteroscopy, new techniques that overcame these issues were needed. Moreover, a sonde enteroscope has no biopsy channel. Yamamoto et al. [1] was the first to develop and use DBE in 2001. Based on the length, channel width, and clinical use, DBE is categorized into three types: diagnostic, therapeutic, and biliary DBE.
Balloon enteroscopy can be used for all small-bowel pathologies including ileus and obstruction. DBE is also very useful for endoscopic retrograde cholangiopancreatography (ERCP) in cases with postsurgical anatomy (Roux-en-Y gastric bypass or other types of gastric bypass surgery) and cases of difficult colonoscopy. The cecal intubation rate was 97% in cases of unsuccessful conventional colonoscopy, and the success rate of DBE-assisted ERCP was 60% to 100% [2,3].
DBE should not be used in patients who are at risk for impaired general condition and perforation. A watery diet and bowel cleansing should be implemented 1 day before the procedure to ensure a successful outcome. Both oral and anal entries can be performed during the same session. The most important advantage of DBE is the applicability of therapeutic interventions during the procedure. However, its disadvantages include a long learning curve, long processing time, and the requirement for patient sedation.
Mehdizadeh et al. [4] performed 127 DBE (63% oral) procedures on a total of 188 patients. The average processing time was 92.4±37.6 minutes, and this period was significantly longer in the first 10 patients (109.1±44.6 minutes; p<0.005). Rectal procedures failed in 31% cases, where the ileum could not be entered. The total rate of diagnosis has been reported as 43%, and the cause of bleeding was unidentified in 51% cases. However, in a study by Yamamoto et al. [5], who improved the technique, the mean procedure duration was 123 minutes and the oral, rectal, and all parts of small bowel could be investigated in 86% of patients over 178 enteroscopy procedures. In studies reported from Europe (70 DBE procedures in 53 patients), the rate of observation of the entire small bowel was 8% and the average procedure time was 72 minutes [6]. In the study conducted by Fukumoto et al. [7], the rate of observation of the entire small bowel was higher, although the difference was not statistically significant.
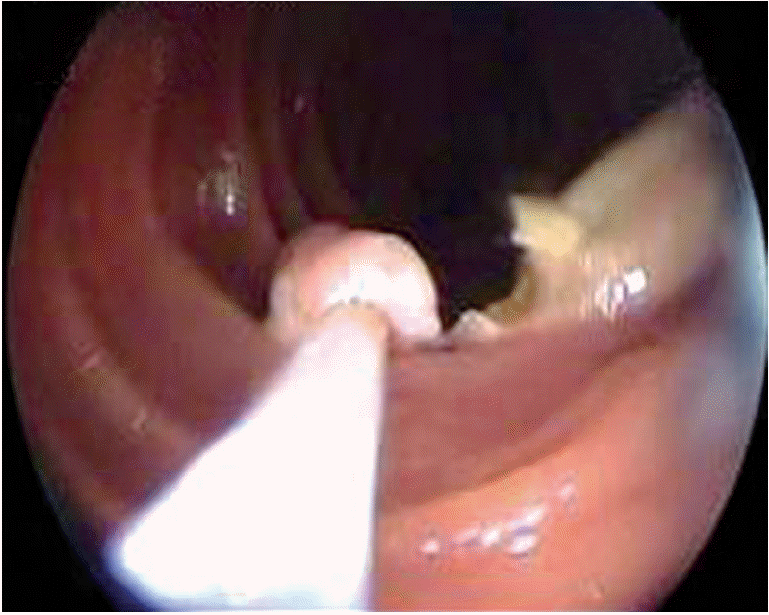
The most important advantage of DBE is that it allows therapeutic approaches such as argon plasma coagulation (APC), polypectomy (Fig. 1), dilatation, foreign body removal, and stent placement during the procedure. May et al. [8] reported that DBE procedures were performed in 60% of 353 patients who were admitted due to bleeding. DBE was found to be diagnostically and therapeutically beneficial in 75% and 67% of these cases, respectively. Although the complication rate for DBE is 3.4%, this rate is reported to be 10.8% in cases of polypectomy (bleeding-perforation) and 0.9% in cases of APC. In a multicenter study that included 2,362 DBE procedures, 709 cases of pancreatitis, five cases of perforation (three APC and two dilatation), and 18 cases of bleeding (13 after polypectomy) were observed. Although the complication rate is greater in patients undergoing interventional procedures, the complication rate remains within acceptable limits [9].
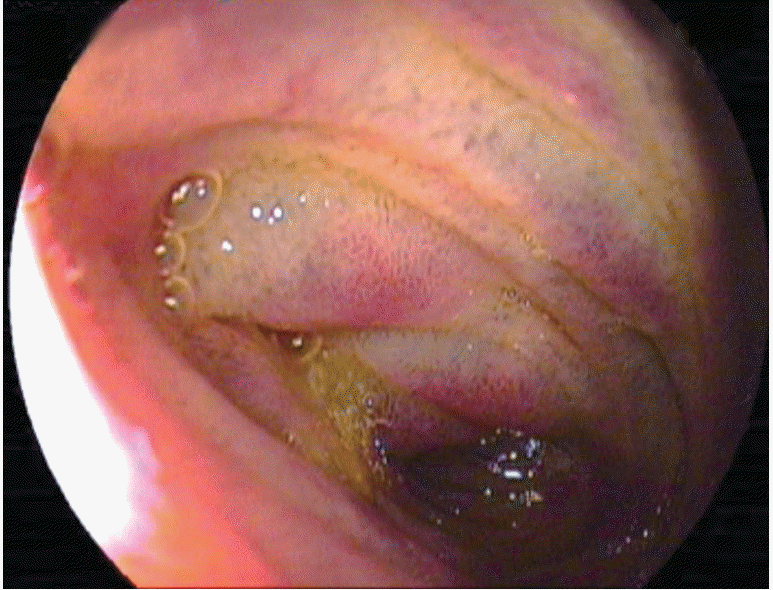
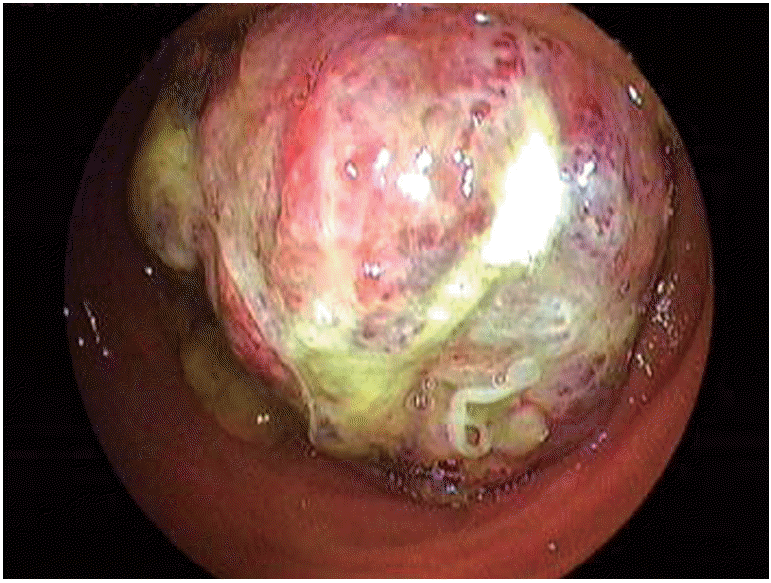
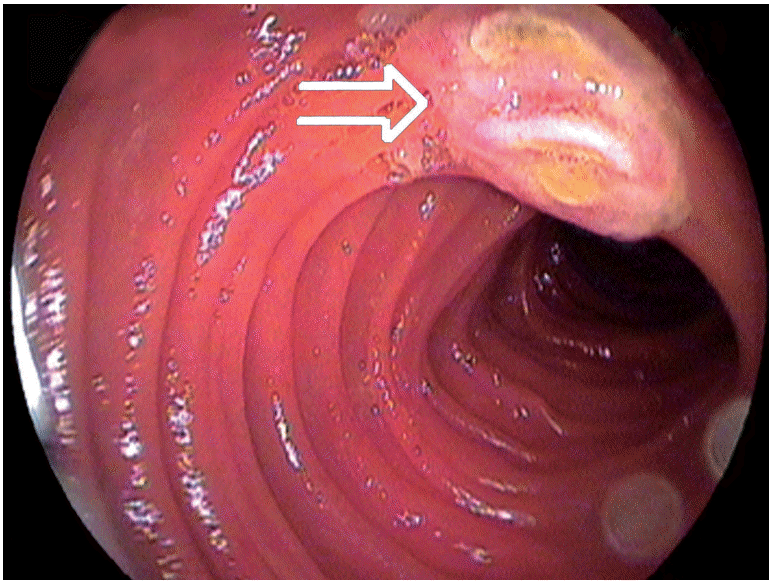
In a study comparing capsule endoscopy (CE) with DBE for gastrointestinal bleeding of unknown cause, it was found that both methods could identify the source of bleeding in some cases(Table 1) [10]. In a meta-analysis study that compared CE with DBE, the diagnosis rates for vascular, inflammatory, and polypoid lesions were similar (Figs. 2-4). The general diagnosis rate of these methods was also similar (CE, 60%; DBE, 57%) [11]. In DBE, although the diagnosis rate of obstruction and bleeding of unknown origin are 81.3% and 80.6%, respectively, the diagnosis rates of diarrhea and abdominal pain are 36.5% and 37.7%, respectively [12]. DBE has been found to be cost-effective for the diagnosis of bleeding of unknown origin [13]. Even though DBE is invasive and its duration of training and procedure time is long, it is usually the first choice as a therapeutic intervention in suitable patients. The diagnosis rates are similar in both methods. Thus, under appropriate conditions, both procedures are good and complementary to each other. Depending on the clinical experience and the available equipment, a different and specific approach for each patient is considered to be the most suitable method.
However, if guidance can be obtained via radiologic methods, either DBE or direct surgery may serve as the first choice. Enteroscopy via the oral route may also be preferred for patients with active bleeding when no radiologic guidance is available [14]. DBE should not be performed in patients who have latex allergy. Instead, single-balloon enteroscopy may be preferable in such cases. In disorders of the small intestine other than bleeding of unknown cause, DBE is primarily preferred after radiologic methods are attempted. The use of oral entry in malabsorption disorders may increase the diagnosis rates. In addition, DBE permits anal entry during the same session. Moreover, DBE can be used to exclude diagnoses in suspicious cases, obtain biopsy specimens from suspicious areas, and achieve dilatation of the stenosis in Crohn disease. In a study that compared DBE with single-balloon enteroscopy, DBE was shown to be three times more effective than total enteroscopic examination. However, single-balloon enteroscopy was reported to be quicker and easier to learn [15]. Moreover, DBE facilitates a greater depth of intubation.
The use of an additional cannula system and CO2 instead of indoor air has also shown to increase the technical achievement rate with DBE, and decompress air during the examination in order to make deep intubation [16,17]. New indications associated with DBE have begun to evolve in clinical practice. Percutaneous jejunostomy is a new therapeutic option for DBE [18,19]. Moreover, the placement of metal stents for malignant stricture of the small intestine is possible [20,21].
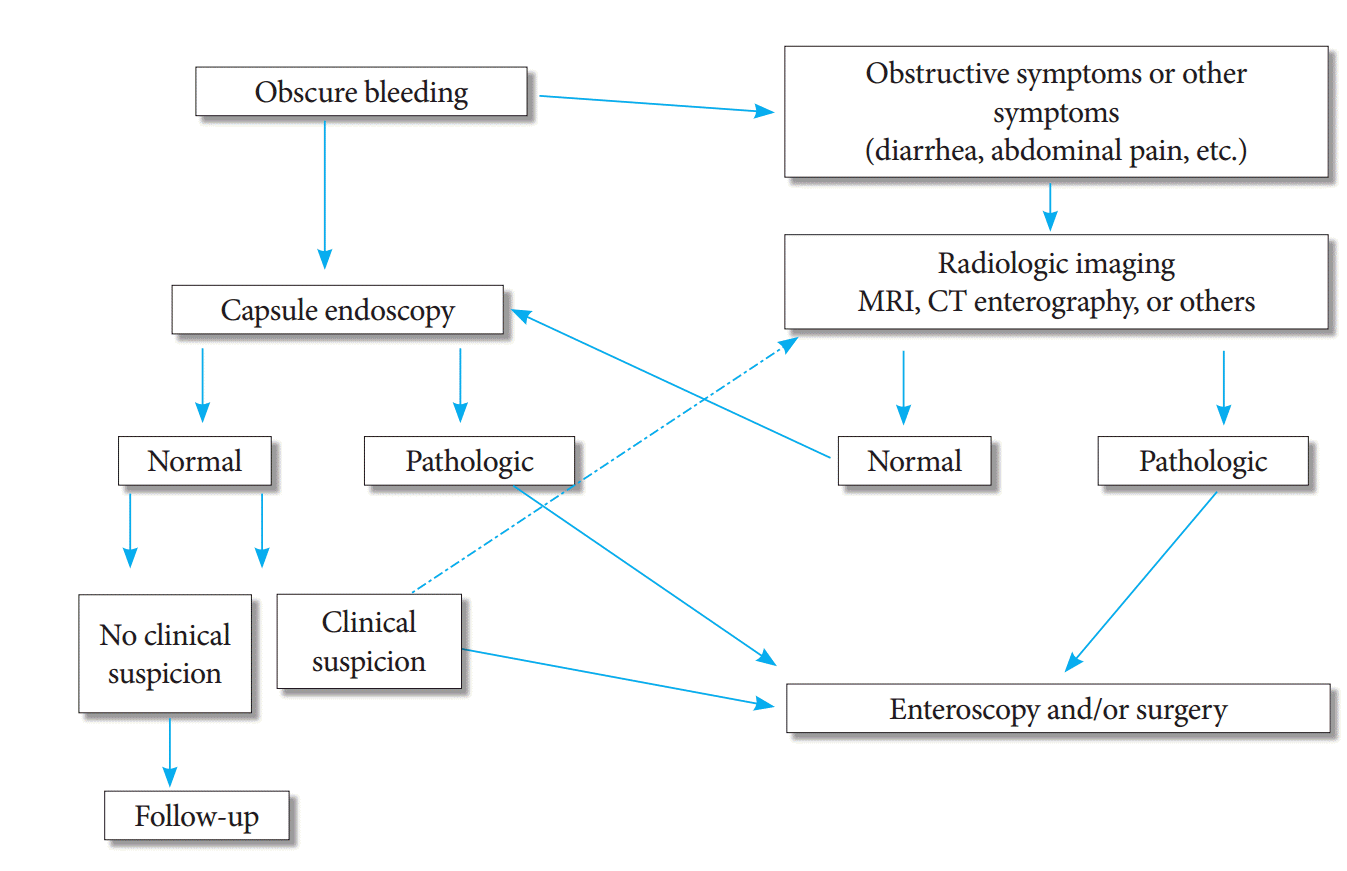
Clinical admissions and alarming symptoms in patients are the main factors through which a treatment method is selected. The method to be followed has been summarized briefly in Fig. 5. Even if the result of CE is negative, enteroscopy or intraoperative enteroscopy should be performed if there is clinical suspicion.
REFERENCES
1. Yamamoto H, Sekine Y, Sato Y, et al. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2001; 53:216–220.

3. Moreels TG, Macken EJ, Roth B, Van Outryve MJ, Pelckmans PA. Cecal intubation rate with the double-balloon endoscope after incomplete conventional colonoscopy: a study in 45 patients. J Gastroenterol Hepatol. 2010; 25:80–83.

4. Mehdizadeh S, Ross A, Gerson L, et al. What is the learning curve associated with double-balloon enteroscopy? Technical details and early experience in 6 U.S. tertiary care centers. Gastrointest Endosc. 2006; 64:740–750.

5. Yamamoto H, Kita H, Sunada K, et al. Clinical outcomes of double-balloon endoscopy for the diagnosis and treatment of small-intestinal diseases. Clin Gastroenterol Hepatol. 2004; 2:1010–1016.

6. Mönkemüller K, Weigt J, Treiber G, et al. Diagnostic and therapeutic impact of double-balloon enteroscopy. Endoscopy. 2006; 38:67–72.

7. Fukumoto A, Tanaka S, Shishido T, Takemura Y, Oka S, Chayama K. Comparison of detectability of small-bowel lesions between capsule endoscopy and double-balloon endoscopy for patients with suspected small-bowel disease. Gastrointest Endosc. 2009; 69:857–865.

8. May A, Nachbar L, Pohl J, Ell C. Endoscopic interventions in the small bowel using double balloon enteroscopy: feasibility and limitations. Am J Gastroenterol. 2007; 102:527–535.

9. Mensink PB, Haringsma J, Kucharzik T, et al. Complications of double balloon enteroscopy: a multicenter survey. Endoscopy. 2007; 39:613–615.

10. Arakawa D, Ohmiya N, Nakamura M, et al. Outcome after enteroscopy for patients with obscure GI bleeding: diagnostic comparison between double-balloon endoscopy and videocapsule endoscopy. Gastrointest Endosc. 2009; 69:866–874.

11. Pasha SF, Leighton JA, Das A, et al. Double-balloon enteroscopy and capsule endoscopy have comparable diagnostic yield in small-bowel disease: a meta-analysis. Clin Gastroenterol Hepatol. 2008; 6:671–676.

12. Zhong J, Ma T, Zhang C, et al. A retrospective study of the application on double-balloon enteroscopy in 378 patients with suspected small-bowel diseases. Endoscopy. 2007; 39:208–215.
13. Gerson L, Kamal A. Cost-effectiveness analysis of management strategies for obscure GI bleeding. Gastrointest Endosc. 2008; 68:920–936.

14. Akyüz Ü, Pata C, Kalayci M, et al. Route selection for double balloon enteroscopy in patients with obscure gastrointestinal bleeding: experience from a single center. Turk J Gastroenterol. 2012; 23:670–675.
15. May A, Färber M, Aschmoneit I, et al. Prospective multicenter trial comparing push-and-pull enteroscopy with the single- and double-balloon techniques in patients with small-bowel disorders. Am J Gastroenterol. 2010; 105:575–581.

16. Ikeya K, Osawa S, Kuriyama S, et al. Decompression side tube-equipped double-balloon enteroscopy extends intubation depth and reduces patient discomfort. Endoscopy. 2012; 44 Suppl 2 UCTN:E256–E257.

17. Soria F, Lopez-Albors O, Morcillo E, et al. Carbon dioxide insufflation safety in double-balloon enteroscopy: an experimental animal study. Dig Endosc. 2013; 25:39–46.

18. Yano T, Yamamoto H, Sunada K, et al. New technique for direct percutaneous endoscopic jejunostomy using double-balloon endoscopy and magnetic anchors in a porcine model. Dig Endosc. 2011; 23:206.

19. Jiang XJ, Song MQ, Xin YN, Gao YQ, Niu ZY, Tian ZB. Endoscopic stenting and concurrent chemoradiotherapy for advanced esophageal cancer: a case-control study. World J Gastroenterol. 2012; 18:1404–1409.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download