Abstract
Severe acute pancreatitis is often complicated by the development of pancreatic fluid collections (PFCs), which may be associated with significant morbidity and mortality. It is crucial to accurately classify these collections as a pseudocyst or walled-off necrosis (WON) given significant differences in outcomes and management. Interventions for PFCs have increasingly shifted to less invasive strategies, with endoscopic ultrasound (EUS)-guided methods being shown to be safer and equally effective as more invasive surgical techniques. In recent years, many new developments have improved the safety and efficacy of EUS-guided interventions, such as the introduction of lumen-apposing metal stents (LAMS), direct endoscopic necrosectomy (DEN) and multiple other adjunctive techniques. Despite these developments, treatment of PFCs, and infected WON in particular, continues to be associated with significant morbidity and mortality. In this article, we discuss the EUS-guided management of PFCs while reviewing the latest developments and controversies in the field. We end by summarizing our own approach to managing PFCs.
The development of pancreatic fluid collections (PFCs) is a frequent complication of moderate-severe and severe acute pancreatitis as well as an occasional feature of chronic pancreatitis. In most cases, PFCs will remain asymptomatic or will resolve on their own over time, and so intervention is frequently unnecessary. However, persistently symptomatic PFCs, and particularly those that become infected, will require drainage and possible debridement. The treatment of PFCs has evolved considerably over the past decade, moving from what was once open surgical management to increasingly minimally invasive techniques, both by surgery and by endoscopy. High rates of complications (64%–95%) and mortality (15%–40%) with surgical treatment of PFCs has spurred the increasing adoption of endoscopic techniques for PFC drainage and debridement [1]. Randomized controlled trials (RCT) have confirmed that an endoscopic approach is safer and associated with lower costs, shorter hospital stay and improved quality of life compared to surgery [2]. In addition, a long awaited and soon to be published RCT from the Dutch Pancreatitis Study Group (TENSION trial) will confirm the superiority of an endoscopic step-up approach over a minimally-invasive surgical step-up approach for the treatment of infected pancreas necrosis (unpublished data).
Recently, endoscopic ultrasound (EUS)-guided drainage has emerged as the leading method for the management of PFCs. EUS-guided drainage has been shown to be safer and to have greater technical success rates compared to conventional transluminal endoscopic drainage methods, and is now considered standard-of-care [3,4]. In this review, we aim to highlight the latest advances and ongoing controversies in the EUS-guided treatment of PFCs.
The first step in the assessment of PFCs is understanding the proper nomenclature outlined in the revised Atlanta classification [5]. This is important because the clinical consequences and treatment approach for PFCs depend entirely on the correct classification of the type of fluid collection. In essence there are two categories of PFCs: a pseudocyst (Fig. 1), which is an encapsulated, fluid-filled collection without solid debris that arises from an acute fluid collection as the result of interstitial edematous pancreatitis; and walled-off necrosis (WON) (Fig. 2), which is an encapsulated collection of both fluid and solid, necrotic debris that arises from an acute necrotic collection as a consequence of necrotizing pancreatitis. The associated mortality risks, likelihood of severe symptoms including infection, probability of spontaneous resolution, and anticipated success rates with endoscopic intervention differ substantially between pseudocysts and WON. For instance, the overall treatment success of endoscopic intervention is significantly higher with pseudocysts compared to WON and the recurrence rate is significantly lower [6,7]. Therefore, the treatment approach for each must be considered separately and the terms “pseudocyst” and PFC should no longer be used interchangeably.
One of the most important considerations when managing patients with PFCs is deciding if and when to intervene. Whereas size greater than 6 cm was previously an indication for drainage, size of the PFC by itself is no longer considered a reason to intervene. In fact, the majority of PFCs tend to resolve spontaneously over time with supportive care and observation. In a prospective observational multicenter study, spontaneous resolution of acute fluid collections was observed in 70% of patients with acute pancreatitis, and only 10% of patients with pseudocysts ultimately required intervention [8]. On the other hand, acute necrotic collections mature into WON in up to 50% of patients. However, only 21% of patients with WON will ever require an intervention and most eventually resolve spontaneously or remain minimally symptomatic over time [9]. Therefore, intervention is truly only required for a minority of patients.
The determining factor that dictates the decision about whether to intervene for a PFC is the presence of significant symptoms, which may include persistent abdominal pain, gastric outlet obstruction, biliary obstruction, fluid leakage due to a disconnected pancreatic duct (PD) and most importantly, infection of the PFC [10-13]. The risk of mortality is particularly significant in patients with infected WON (12%–39%) and therefore, intervention in this context becomes mandatory [14]. It is important to keep in mind that endoscopic interventions for PFCs are associated with potential risks, and therefore, the possibility of procedural complications must be justified by the severity of patients’ symptoms.
The approach to intervening for PFCs can be summarized in 3 steps: delay, drain and if necessary, debride. As much as possible, any intervention for a pseudocyst or WON should be delayed as long as clinical circumstances allow in order to give time for a mature wall to develop around the collection. If the patient is septic and source control is required prior to maturation of the PFC, percutaneous catheter drainage is an appropriate temporizing measure. Otherwise drainage should be delayed for at least 4 weeks or longer until the PFC is encapsulated. Surgical series have clearly demonstrated that earlier intervention for WON was associated with increased mortality when compared to delayed intervention, with improved outcomes the longer the delay between admission and intervention [15].
It is critical when preparing to intervene for a PFC that it is determined whether the patient has a pseudocyst or WON. Computed tomography (CT) scan notoriously underestimates the presence and extent of solid necrotic debris within a collection compared to magnetic resonance imaging (MRI) or EUS. One study found that CT identified the presence of solid necrotic debris in PFCs in only 32% of patients compared to 92% with EUS (p<0.001) [16]. Hence, careful assessment of a PFC by EUS or MRI to clarify whether it is WON is crucial prior to embarking on endoscopic therapy to ensure appropriate patient selection and choice of intervention strategy. Endoscopic drainage for pseudocysts is relatively straightforward with high rates of treatment success irrespective of the type or size of stents inserted, complications are rare and the need for repeat procedures uncommon. In contrast, the treatment of WON is much more difficult, outcomes may vary based on technique, choice of stents, use of additional irrigation, and may not respond to initial drainage, in which case debridement of necrotic tissue from within the collection is often necessary. Such debridement, termed direct endoscopic necrosectomy (DEN), remains somewhat controversial since it carries significantly increased complication rates compared to creation of the drainage tract by EUS-guided cyst-gastrostomy or cyst-duodenostomy. In a retrospective analysis of the treatment outcomes for PFCs at the University of Alabama in an era pre-dating the use of metal stents, treatment success rates (63% vs. 94%, p<0.0001), need for repeat procedures (32% vs. 10%), hospital length-of-stay (5 days vs. 2 days, p<0.0001) and complication rates (16% vs. 5%, p=0.02) were all inferior for WON compared to pseudocysts, illustrating that the treatment of WON is more challenging [6]. In the coming sections, we will discuss important considerations when intervening for patients with WON, and to a lesser extent, for patients with pseudocysts.
EUS-guided drainage of pseudocysts and WON involves creation of a fistula tract between the lumen of the gastrointestinal (GI) tract, typically the stomach (cyst-gastrostomy) or duodenum (cyst-duodenostomy), and the PFC. However, this trans-luminal drainage is only effective if the fistula tract is kept patent by placement of stents through the tract under EUS, fluoroscopic and endoscopic guidance. Traditionally, double pigtail plastic stents (PSs) have been used for this purpose, but fully covered self-expanding metal stents (FcSEMS) have been increasingly used instead, and recently lumen-apposing metal stents (LAMS) that are specifically designed for PFC drainage have been introduced (Table 1).
The overall efficacy of transmural stenting for pseudocysts using PS is more than 90%.2,17 This is true regardless of the number or size (7 F vs. 10 F) of the PS used [18]. On the other hand, the overall treatment success of PS is significantly lower for drainage of WON compared to that for pseudocysts [6]. This may be due to the small diameter of PS and the presence of solid debris in WON that is more difficult to drain through the fistula tract. This increases the risk of stent occlusion with resultant secondary infection of the WON [19].
Consequently, straight biliary FcSEMS have been tried in patients with PFCs given theoretical advantages of improved drainage due to larger stent caliber. A number of studies assessed the efficacy of these metal stents for pseudocyst drainage and the overall treatment success was excellent (85%–95%) [20,21]. Given these encouraging results, the next question was whether FcSEMS provided any advantage over PS. A retrospective study found FcSEMS to be superior both in terms of resolution of the PFC and in adverse events when compared to PS [19]. However, a randomized study failed to demonstrate superiority of FcSEMS over PS for pseudocyst drainage (clinical success 87% vs. 91%, p=0.97) [22]. The only advantage of FcSEMS was shorter procedure time (15 min vs. 29.5 min, p<0.01). This was further confirmed in a meta-analysis that found no difference in overall treatment success rates between patients with pseudocysts treated with PS or with metal stents (85% vs. 83%, respectively) [23]. It also failed to show a better safety profile or lower recurrence rate with metal stents. On the other hand, FcSEMS do seem to have superior rates of treatment success compared to PS when used to drain WON [2,24]. However, the main disadvantage of the straight FcSEMS is stent migration, with rates as high as 15% having been reported [25].
To address some of the challenges encountered with PSs and the straight FcSEMS, LAMS with a unique “dumbbell” design that bring the walls of the lumen and the PFC close together were introduced (Fig. 3). Treatment success rates approaching 100% have been achieved using these stents to drain pseudocysts [26,27], although its unclear whether LAMS would ultimately be superior to PS since success rates for pseudocysts are so high regardless of stent choice. What is more clinically relevant is the potential for using LAMS to achieve improved outcomes in the drainage of WON. So far, the early data have been impressive, with overall technical success rates exceeding 90% and clinical success rates of 85%–91%, with many patients achieving complete resolution of WON without need for DEN. Complications have been observed in 10%–15% of patients, while very few patients have gone on to require surgery [27,28]. Furthermore, migration of LAMS occurred in only 5% of patients [29], and their insertion required significantly shorter procedure times when compared to PS (25 min vs. 43 min, p=0.01) [30].
The choice of stent used to drain WON remains one of the most actively researched areas in the endoscopic management of PFCs. A systematic review that included 17 studies involving 881 patients compared the efficacy of plastic (both straight and double-pigtail) and metal stents (both straight FcSEMS and LAMS) in the management of PFCs [23]. The authors found that overall treatment success in patients with WON was similar between those treated with plastic and with metal stents (70% vs. 78%, respectively). In addition, there was no difference in adverse events or rates of recurrence. However, this systematic review was comprised of non-comparative cohort studies that each used a single type of stent, with the majority of studies being of low quality.
Since the publication of this systematic review, multiple studies have demonstrated improved outcomes for the treatment of WON when using metal stents, with fewer endoscopic necrosectomy sessions required to achieve resolution, fewer adverse events, shorter hospital stay and reduced need for salvage surgery [27,31]. Furthermore, a recent study found superior resolution rates of WON at 6 months follow-up when drainage had been performed with metal stents (both straight FcSEMS and LAMS) than with PS [27]. However, to date no significant difference in efficacy has been shown between straight FcSEMS and the new LAMS, with long-term success rates of 95% vs. 90%, respectively [32].
Based on the limited available evidence, metal stents (either straight FcSEMS or LAMS) seem to be more effective than PS for drainage of WON and may reduce the need for DEN. The use of such stents should be considered first-line when draining/debriding WON, however randomized comparative trials are still needed to prove this concept. In contrast, there is high quality evidence that shows no advantage for metal stents over PS for drainage of pseudocysts, albeit with shorter procedure time. Given the increased costs and possibly increased risk of bleeding using metal stents, PS remain the current recommendation for pseudocyst drainage.
A number of complications may occur when performing endoscopic management of PFCs including bleeding, perforation, secondary infection and stent migration [33]. The use of EUS may help to reduce the risk of bleeding by visualizing any intervening vessels. One prospective study reported a 13% rate of bleeding with conventional endoscopic drainage compared to no bleeding with EUS-guided interventions [3]. However, even with EUS guidance, bleeding remains an important adverse event, particularly when metal stents are used [27]. Stent migration is a well-described complication for both PS and FcSEMS, which can occur externally into the GI tract or internally into the PFC. The risk of stent migration ranges between 1%–15% [6,25]. External stent migration can usually be easily managed by endoscopic removal of the stent. However, internal migration of a stent into the PFC cavity may pose a more difficult situation that can result in bleeding if the stent erodes into a large blood vessel. However, internal migration of stents can also be effectively dealt with by dilation of the fistula tract and endoscopic retrieval of the stent from within the PFC using a similar technique as when performing DEN. Infection of PFCs after endoscopic intervention can occur in up to 20% of cases, often resulting in need for DEN or even surgical intervention. Indeed, a recent retrospective study showed a higher rate of adverse events with PS compared to FcSEMS (31% vs. 16%, p=0.006), predominantly due to secondary infection that occurs when the stents become blocked and/or the drainage tract closes [19]. As a result, patients with PS were 2.9 times more likely to experience an adverse event compared to those with FcSEMS (odds ratio, 2.9; 95% confidence interval, 1.4–6.3) on multivariable analysis.
Despite the promising early data using LAMS, one study raised some serious concerns about safety issues. In this study, Bang et al. compared the efficacy of LAMS with double-pigtail PS for the management of WON [34]. In this ongoing study, 21 patients were randomized to either LAMS (12 patients) or PS (9 patients). An interim audit revealed a high rate of complications (50%) in the LAMS group, compared to 0% with PS. These complications included delayed bleeding, buried stent syndrome and stent-induced biliary strictures resulting in jaundice. Interestingly, all were delayed adverse events that occurred more than 3 weeks after the index intervention. However, it is unclear whether these preliminary results reflect the true risk of complications, since the rate of adverse events were much higher than those described in previous studies [27,28]
Once EUS-guided drainage of a PFC is established, a trans-nasal catheter can be placed over a wire through the fistula track and into the PFC to provide irrigation within the cavity. Normal saline or sterile water is flushed via the nasocystic tube into the PFC (usually for WON) with the aim of improving drainage. This may be particularly useful when the collection is infected or filled with a significant amount of necrotic debris. In a retrospective study, the use of a nasocystic tube alongside PSs in patients with WON was shown to result in higher short-term success (85% vs. 63%) and decreased stent occlusion rates (13% vs. 33%) compared to the use of PS alone [35]. Furthermore, a recently published Cochrane Review found that the use of a nasocystic tube with EUS-guided drainage was associated with lower adverse events and shorter hospital stay than when EUS-guided drainage was performed alone [36]. However, it is unclear if a nasocystic tube will continue to offer any advantage when used together with one of the newer LAMS [27].
Based on the current evidence we highly recommend the use of a nasocystic tube to facilitate irrigation of WON, particularly when there are signs of infection, and especially in patients treated with PSs. On the other hand, we believe the majority of pseudocysts can be drained successfully using transmural stents without the need for a nasocystic tube.
A longstanding area of uncertainty is whether patients with PFCs who are treated with endoscopic transluminal drainage by EUS also require transpapillary drainage by endoscopic retrograde cholangiopancreatography (ERCP). Disruption of the PD commonly occurs as the result of pancreas necrosis, leaving a functioning body or tail that is disconnected from the head of the pancreas, with pancreas secretions unable to reach the papilla. This disruption results in ongoing leakage of pancreatic exocrine secretions leading to persistence or recurrence of a PFC. An intuitive solution is the placement of a transpapillary stent in the PD to bridge the site of the leak and to facilitate preferential drainage via the PD. However, it has been unclear whether doing so results in improved treatment success rates for pseudocysts or WON.
Initial studies supported this hypothesis with improved outcomes observed in patients treated with combined transmural and transpapillary approaches combined to transmural drainage alone [37]. However, the benefit appeared to be limited to patients with partial (rather than complete) PD disruption, possibly due to the unlikelihood of successfully bridging a completely disrupted PD [38]. Not surprisingly, patients without PD disruption failed to show any advantage with combination therapy [39]. Further data came from a recent multicenter retrospective study that included 174 patients who underwent EUS-guided drainage of pseudocysts that evaluated outcomes using a combined transluminal/transpapillary approach (n=79) compared to a transmural approach alone (n=95). Technical success was higher in the transmural group (97% vs. 44%, p=0.0001) with no difference in adverse events (15% vs. 14%, p=0.23). More importantly, there was no difference in the long-term rates of symptomatic resolution (69% vs. 62%, p=0.61) or complete radiological resolution (71% vs. 67%, p=0.79) between transmural and combined approaches, respectively. In fact, attempts at transpapillary drainage were negatively associated with long-term resolution [40]. Therefore, based on this latest evidence, routine transpapillary drainage for PFCs is not recommended. However, ERCP should be considered if the patient has an obstructive process in the PD (e.g., stone or stricture) to treat the underlying obstructive abnormality.
One important, yet unresolved, issue when draining PFCs is the duration of stenting. PFCs have been shown to recur in 10%–38% of patients in the first year after stent removal [40,41]. In theory, keeping the stent will maintain patency of the cystenterostomy tract and prevent PFC recurrence, but there are no data to confirm the long-term safety of leaving these stents in place. Prolonged stent placement (using PSs) was shown to be superior to protocolized stent removal by a prospective trial that randomized 28 patients to removal of the stents 2 weeks after PFC resolution or to keeping them in place. At 14 months, the recurrence rate was 38% in the stent removal group compared to no recurrence in the long-term stent group, with no complications experienced by patients with prolonged stenting [41]. However, the patients who should benefit from prolonged transluminal stenting are those with a viable body or tail of the pancreas with a disrupted PD. In this “disconnected pancreatic duct syndrome (DPDS)” pancreatic secretions from the disconnected body and/or tail leak from the disrupted PD, resulting in persistence or recurrence of a pseudocyst. In this circumstance, long-term or even permanent drainage via a cyst-gastrostomy fistula tract is necessary, in which case indefinite PS placement is recommended. This approach has been demonstrated by multiple centers to be a safe and effective solution in greater than 90% of patients with DPDS [42-44]. A significant consideration when deciding upon the duration of transluminal stent placement is whether double pigtail PSs or a metal stent are in place. Some concerns have been raised about increased risks of delayed bleeding from a collapsed WON collection when a metal stent is in place, which is why we advocate stent removal after the PFC resolves if a FcSEMS is in place, except for cases of DPDS in which case we replace the FcSEMS with plastic double pigtail stents.
As mentioned previously, symptomatic WON (especially infected WON) has significant morbidity and mortality [14]. However, successful treatment of WON via transmural drainage is often difficult, with rates of success as low as 25% being reported using traditional transmural PS and a nasocystic drain [39]. A meta-analysis summarizing the results from 12 studies involving 481 patients with infected WON who were treated with percutaneous or endoscopic drainage found a pooled treatment success rate of only 59% [45]. This leaves a significant group of patients with infected WON not responding to drainage procedures that require more aggressive debridement of the necrotic debris within the collection via DEN (or surgery).
DEN consists of debridement of WON using a gastroscope that is inserted directly into the collection via the stomach or duodenum through the cyst-gastrostomy or cyst-duodenostomy fistula tract. The tract is dilated to enable passage of the endoscope and then the necrotic debris is slowly removed from the WON and pulled back into the lumen using a variety of endoscopic tools (Fig. 4). One of the earliest studies to report outcomes with DEN was the GEPARD trial [46], which was a multicenter study of 93 patients with WON who underwent transluminal endoscopic debridement of peri-pancreatic and pancreatic necrosis, achieving an 80% success rate. Despite these encouraging results, complications were common, occurring in 26% of patients, with 7.5% mortality. Similar outcomes have been observed in subsequent studies [47,48], and a recent meta-analysis found pooled rates of treatment success, complications and mortality of 81%, 35% and 6%, respectively [49]. Reported complications include perforation, air embolism, and bleeding, which occurs in 3%–21% of patients [46,47,50]. Therefore, despite the fact that DEN may contribute to accelerated patient recovery and clinical resolution of infected WON, the morbidity and mortality associated with the procedure should limit its use to circumstances in which patients have failed to improve after appropriate transluminal drainage, with a target treatment endpoint of clinical resolution of significant symptoms, not radiological resolution.
Given the significant morbidity and mortality associated with DEN, a number of less invasive endoscopic interventions have been described that aim to remove the need for actual debridement of the WON by facilitating transluminal drainage. The first of these methods is simply the use of metal rather than PSs given the expectation, and some evidence, that FcSEMS are more effective than PS in draining WON. Some recent studies have demonstrated successful resolution of WON using metal stents without the need for DEN in 20%–40% of patients [27,51]. Therefore, FcSEMS may help to avoid DEN in some patients and may be used to provide access into the collection to perform DEN in patients who fail to improve. Another technique used to optimize WON drainage is the “Multiple Transluminal Gateway Technique (MTGT)” described by Varadarajulu et al. [52]. In this method, multiple transluminal fistulae are created in addition to the primary cyst-gastrostomy or cyst-duodenostomy drainage tract, with PSs placed in each to maintain tract patency. A nasocystic tube is used to irrigate the WON through one tract while the additional drainage tracts serve as conduits for efflux of necrotic debris. In their study of 60 patients with WON who were treated with either the MTGT approach or with standard transmural drainage using PSs, the overall treatment success was 92% for MTGT compared to 52% with the standard approach. There were no adverse events in the MTGT group compared to a 10% complication rate in the conventional drainage group. While further study is needed, MTGT appears to be a promising technique to reduce the need for DEN.
Another promising adjunctive method is the combination of transluminal and percutaneous drainage catheters to achieve “dual modality drainage (DMD)” [53,54]. DMD involves placement of a CT-guided percutaneous drain followed immediately by EUS-guided placement of transmural stents into the WON. Once the transmural stents are in place, the external drain is opened and flushed routinely. The percutaneous drain is then progressively up-sized (up to 24 F) to facilitate increased drainage. Once the PFC resolves, the percutaneous drain is capped and removed. The transmural stents are usually removed once repeat imaging confirms that the WON has resolved, or can be left in place permanently in cases of a residual functioning body or tail with a disconnected PD. The studies supporting DMD have all been from one U.S. center but with high rates of success at avoiding DEN or surgical necrosectomy.
Another novel technique used to facilitate drainage from WON is endoscopic irrigation using a high-flow water jet. Once the standard transluminal fistula tract is created under EUS guidance and a FcSEMS is in place, a gastroscope is positioned in the opening of the stent without actually entering into the WON. The cavity is then irrigated with 700–1,200 mL of normal saline using a water jet via a tapered-tip 5 F biliary catheter that is passed through the metal stent while continuous aspiration of fluid from the stomach is simultaneously performed through the endoscope suction channel. This procedure is repeated every few days until signs of sepsis from infected WON or an ongoing systemic inflammatory response resolve. Importantly, the gastroscope is not advanced directly into the WON cavity and no mechanical debridement is performed, which is presumably safer and distinguishes it from DEN. In its initial description, this method was successful in achieving resolution of WON without the need for DEN in 82% of patients with no major complications [55]. However, this is based on a small retrospective single-center study and larger, prospective multi-center studies are needed to validate these encouraging preliminary results.
EUS-guided intervention has become an important component of the treatment of PFCs and currently is the first line approach for most patients in centers with experienced endoscopists. Recent advances have significantly improved the efficacy and safety of endoscopic PFC drainage procedures, with LAMS in particular holding substantial promise for the improved and simplified treatment of these collections. The endoscopic management of pseudocysts is relatively straight forward, with high rates of success regardless of what type of stent is used and whether nasocystic irrigation is performed. For this reason we advocate the use of PSs and no nasocystic catheter. On the other hand, WON remains a therapeutic challenge that poses significant morbidity and mortality, particularly once infected. In these cases we believe that EUS-guided placement of a FcSEMS, and in particular a LAMS, may provide clinical benefit over the use of double pigtail PSs. We also continue to advocate irrigation of infected WON with a nasocystic tube, insertion of a percutaneous catheter to facilitate drainage in cases of particularly large collections with extensive solid debris, and DEN when patients fail to respond to drainage procedures alone. In the near future, we anticipate further research will determine the cost-benefit trade off between metal and PSs, and in particular, the significantly increased costs of LAMS compared to straight biliary FcSEMS. In addition, we expect further work will clarify the need for, and benefit from, additional techniques to facilitate drainage, as well as improved methods for the most effective and safest debridement of infected necrotic material when clinically necessary.
REFERENCES
1. Gomatos IP, Halloran CM, Ghaneh P, et al. Outcomes from minimal access retroperitoneal and open pancreatic necrosectomy in 394 patients with necrotizing pancreatitis. Ann Surg. 2016; 263:992–1001.

2. Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, Wilcox CM. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013; 145:583–590.e1.

3. Varadarajulu S, Christein JD, Tamhane A, Drelichman ER, Wilcox CM. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos). Gastrointest Endosc. 2008; 68:1102–1111.

4. Park DH, Lee SS, Moon SH, et al. Endoscopic ultrasound-guided versus conventional transmural drainage for pancreatic pseudocysts: a prospective randomized trial. Endoscopy. 2009; 41:842–848.

5. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013; 62:102–111.

6. Varadarajulu S, Bang JY, Phadnis MA, Christein JD, Wilcox CM. Endoscopic transmural drainage of peripancreatic fluid collections: outcomes and predictors of treatment success in 211 consecutive patients. J Gastrointest Surg. 2011; 15:2080–2088.

7. Baron TH, Harewood GC, Morgan DE, Yates MR. Outcome differences after endoscopic drainage of pancreatic necrosis, acute pancreatic pseudocysts, and chronic pancreatic pseudocysts. Gastrointest Endosc. 2002; 56:7–17.

8. Cui ML, Kim KH, Kim HG, et al. Incidence, risk factors and clinical course of pancreatic fluid collections in acute pancreatitis. Dig Dis Sci. 2014; 59:1055–1062.

9. Sarathi Patra P, Das K, Bhattacharyya A, et al. Natural resolution or intervention for fluid collections in acute severe pancreatitis. Br J Surg. 2014; 101:1721–1728.

10. Baron TH, Morgan DE. The diagnosis and management of fluid collections associated with pancreatitis. Am J Med. 1997; 102:555–563.
11. Yeo CJ, Bastidas JA, Lynch-Nyhan A, Fishman EK, Zinner MJ, Cameron JL. The natural history of pancreatic pseudocysts documented by computed tomography. Surg Gynecol Obstet. 1990; 170:411–417.
12. Bradley EL, Clements JL Jr, Gonzalez AC. The natural history of pancreatic pseudocysts: a unified concept of management. Am J Surg. 1979; 137:135–141.

13. Gouyon B, Lévy P, Ruszniewski P, et al. Predictive factors in the outcome of pseudocysts complicating alcoholic chronic pancreatitis. Gut. 1997; 41:821–825.

14. van Brunschot S, Bakker OJ, Besselink MG, et al. Treatment of necrotizing pancreatitis. Clin Gastroenterol Hepatol. 2012; 10:1190–1201.

15. Mier J, León EL, Castillo A, Robledo F, Blanco R. Early versus late necrosectomy in severe necrotizing pancreatitis. Am J Surg. 1997; 173:71–75.

16. Medarapalem JB, Appasani S, Gulati A, et al. Characterization of fluid collections using quantification of solid debris in acute pancreatitis - a comparative study of EUS vs. CT for prediction of intervention. Gastrointest Endosc. 2014; 79(Suppl 5):AB445.
17. Zheng M, Qin M. Endoscopic ultrasound guided transgastric stenting for the treatment of traumatic pancreatic pseudocyst. Hepatogastroenterology. 2011; 58:1106–1109.

18. Bang JY, Wilcox CM, Trevino JM, et al. Relationship between stent characteristics and treatment outcomes in endoscopic transmural drainage of uncomplicated pancreatic pseudocysts. Surg Endosc. 2014; 28:2877–2883.

19. Sharaiha RZ, DeFilippis EM, Kedia P, et al. Metal versus plastic for pancreatic pseudocyst drainage: clinical outcomes and success. Gastrointest Endosc. 2015; 82:822–827.

20. Talreja JP, Shami VM, Ku J, Morris TD, Ellen K, Kahaleh M. Transenteric drainage of pancreatic-fluid collections with fully covered self-expanding metallic stents (with video). Gastrointest Endosc. 2008; 68:1199–1203.

21. Fabbri C, Luigiano C, Cennamo V, et al. Endoscopic ultrasound-guided transmural drainage of infected pancreatic fluid collections with placement of covered self-expanding metal stents: a case series. Endoscopy. 2012; 44:429–433.

22. Lee BU, Song TJ, Lee SS, et al. Newly designed, fully covered metal stents for endoscopic ultrasound (EUS)-guided transmural drainage of peripancreatic fluid collections: a prospective randomized study. Endoscopy. 2014; 46:1078–1084.

23. Bang JY, Hawes R, Bartolucci A, Varadarajulu S. Efficacy of metal and plastic stents for transmural drainage of pancreatic fluid collections: a systematic review. Dig Endosc. 2015; 27:486–498.
24. Tarantino I, Barresi L, Fazio V, Di Pisa M, Traina M. EUS-guided self-expandable stent placement in 1 step: a new method to treat pancreatic abscess. Gastrointest Endosc. 2009; 69:1401–1403.

25. Penn DE, Draganov PV, Wagh MS, Forsmark CE, Gupte AR, Chauhan SS. Prospective evaluation of the use of fully covered self-expanding metal stents for EUS-guided transmural drainage of pancreatic pseudocysts. Gastrointest Endosc. 2012; 76:679–684.

26. Itoi T, Binmoeller KF, Shah J, et al. Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos). Gastrointest Endosc. 2012; 75:870–876.

27. Siddiqui AA, Kowalski TE, Loren DE, et al. Fully covered self-expanding metal stents versus lumen-apposing fully covered self-expanding metal stent versus plastic stents for endoscopic drainage of pancreatic walled-off necrosis: clinical outcomes and success. Gastrointest Endosc. 2017; 85:758–765.

28. Shah RJ, Shah JN, Waxman I, et al. Safety and efficacy of endoscopic ultrasound-guided drainage of pancreatic fluid collections with lumen-apposing covered self-expanding metal stents. Clin Gastroenterol Hepatol. 2015; 13:747–752.

29. Sharaiha RZ, Tyberg A, Khashab MA, et al. Endoscopic therapy with lumen-apposing metal stents is safe and effective for patients with pancreatic walled-off necrosis. Clin Gastroenterol Hepatol. 2016; 14:1797–1803.
30. Gornals JB, De la Serna-Higuera C, Sánchez-Yague A, Loras C, Sánchez-Cantos AM, Pérez-Miranda M. Endosonography-guided drainage of pancreatic fluid collections with a novel lumen-apposing stent. Surg Endosc. 2013; 27:1428–1434.

31. Bapaye A, Dubale NA, Sheth KA, et al. Endoscopic ultrasonography-guided transmural drainage of walled-off pancreatic necrosis: comparison between a specially designed fully covered bi-flanged metal stent and multiple plastic stents. Dig Endosc. 2017; 29:104–110.

32. Vazquez-Sequeiros E, Baron TH, Pérez-Miranda M, et al. Evaluation of the short- and long-term effectiveness and safety of fully covered self-expandable metal stents for drainage of pancreatic fluid collections: results of a Spanish nationwide registry. Gastrointest Endosc. 2016; 84:450–457.e2.

33. Varadarajulu S, Christein JD, Wilcox CM. Frequency of complications during EUS-guided drainage of pancreatic fluid collections in 148 consecutive patients. J Gastroenterol Hepatol. 2011; 26:1504–1508.

34. Bang JY, Hasan M, Navaneethan U, Hawes R, Varadarajulu S. Lumen-apposing metal stents (LAMS) for pancreatic fluid collection (PFC) drainage: may not be business as usual. Gut. 2016; Aug. 31. [Epub]. https://doi.org/10.1136/gutjnl-2016-312812.

35. Siddiqui AA, Dewitt JM, Strongin A, et al. Outcomes of EUS-guided drainage of debris-containing pancreatic pseudocysts by using combined endoprosthesis and a nasocystic drain. Gastrointest Endosc. 2013; 78:589–595.

36. Gurusamy KS, Pallari E, Hawkins N, Pereira SP, Davidson BR. Management strategies for pancreatic pseudocysts. Cochrane Database Syst Rev. 2016; 4:CD011392.

37. Trevino JM, Tamhane A, Varadarajulu S. Successful stenting in ductal disruption favorably impacts treatment outcomes in patients undergoing transmural drainage of peripancreatic fluid collections. J Gastroenterol Hepatol. 2010; 25:526–531.

38. Shrode CW, Macdonough P, Gaidhane M, et al. Multimodality endoscopic treatment of pancreatic duct disruption with stenting and pseudocyst drainage: how efficacious is it? Dig Liver Dis. 2013; 45:129–133.

39. Hookey LC, Debroux S, Delhaye M, Arvanitakis M, Le Moine O, Devière J. Endoscopic drainage of pancreatic-fluid collections in 116 patients: a comparison of etiologies, drainage techniques, and outcomes. Gastrointest Endosc. 2006; 63:635–643.

40. Yang D, Amin S, Gonzalez S, et al. Transpapillary drainage has no added benefit on treatment outcomes in patients undergoing EUS-guided transmural drainage of pancreatic pseudocysts: a large multicenter study. Gastrointest Endosc. 2016; 83:720–729.
41. Arvanitakis M, Delhaye M, Bali MA, et al. Pancreatic-fluid collections: a randomized controlled trial regarding stent removal after endoscopic transmural drainage. Gastrointest Endosc. 2007; 65:609–619.

42. Devière J, Bueso H, Baize M, et al. Complete disruption of the main pancreatic duct: endoscopic management. Gastrointest Endosc. 1995; 42:445–451.

43. Pelaez-Luna M, Vege SS, Petersen BT, et al. Disconnected pancreatic duct syndrome in severe acute pancreatitis: clinical and imaging characteristics and outcomes in a cohort of 31 cases. Gastrointest Endosc. 2008; 68:91–97.

44. Varadarajulu S, Wilcox CM. Endoscopic placement of permanent indwelling transmural stents in disconnected pancreatic duct syndrome: does benefit outweigh the risks? Gastrointest Endosc. 2011; 74:1408–1412.

45. Moul VP, Sreenivas V, Garg PK. Efficacy of conservative treatment, without necrosectomy, for infected pancreatic necrosis: a systematic review and meta-analysis. Gastroenterology. 2013; 144:333–340.e2.

46. Seifert H, Biermer M, Schmitt W, et al. Transluminal endoscopic necrosectomy after acute pancreatitis: a multicentre study with long-term follow-up (the GEPARD study). Gut. 2009; 58:1260–1266.

47. Yasuda I, Nakashima M, Iwai T, et al. Japanese multicenter experience of endoscopic necrosectomy for infected walled-off pancreatic necrosis: the JENIPaN study. Endoscopy. 2013; 45:627–634.

48. Gardner TB, Chahal P, Papachristou GI, et al. A comparison of direct endoscopic necrosectomy with transmural endoscopic drainage for the treatment of walled-off pancreatic necrosis. Gastrointest Endosc. 2009; 69:1085–1094.

49. van Brunschot S, Fockens P, Bakker OJ, et al. Endoscopic transluminal necrosectomy in necrotising pancreatitis: a systematic review. Surg Endosc. 2014; 28:1425–1438.

50. Gardner TB, Coelho-Prabhu N, Gordon SR, et al. Direct endoscopic necrosectomy for the treatment of walled-off pancreatic necrosis: results from a multicenter U.S. series. Gastrointest Endosc. 2011; 73:718–726.

51. Rinninella E, Kunda R, Dollhopf M, et al. EUS-guided drainage of pancreatic fluid collections using a novel lumen-apposing metal stent on an electrocautery-enhanced delivery system: a large retrospective study (with video). Gastrointest Endosc. 2015; 82:1039–1046.

52. Varadarajulu S, Phadnis MA, Christein JD, Wilcox CM. Multiple transluminal gateway technique for EUS-guided drainage of symptomatic walled-off pancreatic necrosis. Gastrointest Endosc. 2011; 74:74–80.

53. Ross A, Gluck M, Irani S, et al. Combined endoscopic and percutaneous drainage of organized pancreatic necrosis. Gastrointest Endosc. 2010; 71:79–84.

Fig. 1.
Computed tomography (CT) scan of a patient with a pseudocyst. The pseudocyst is surrounded by a mature wall and is free of any solid debris.
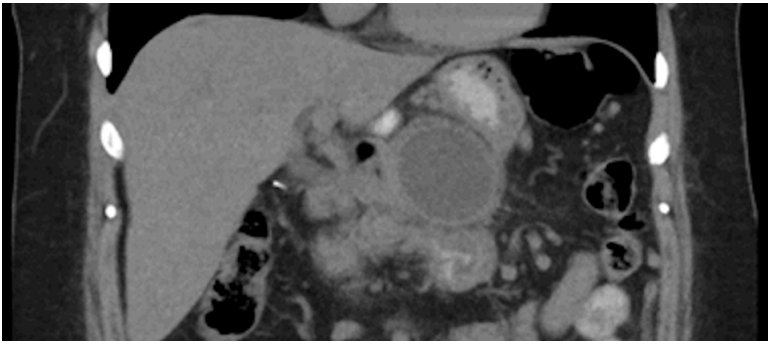
Fig. 2.
Computed tomography (CT) scan of a patient with walled-off necrosis (WON). Note the heterogeneous appearance of the collection that contains solid, necrotic debris.
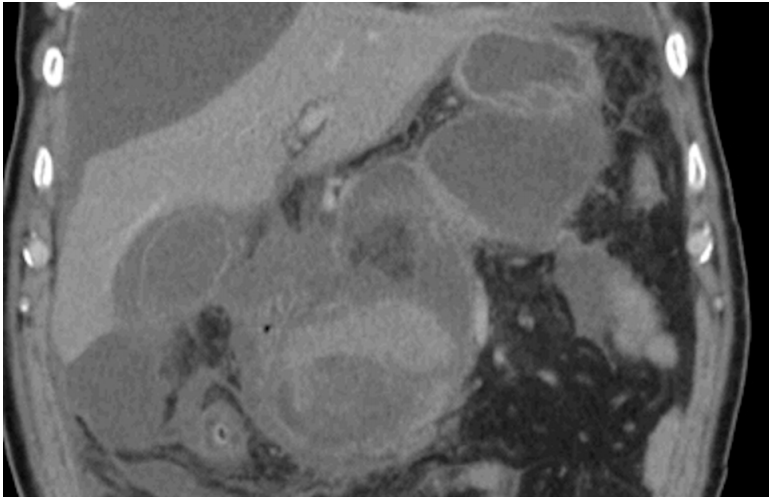
Fig. 3.
Patient with symptomatic walled-off necrosis (WON) with trans-gastric lumen-apposing metal stents (LAMS) inserted under endoscopic ultrasound (EUS)-guidance.
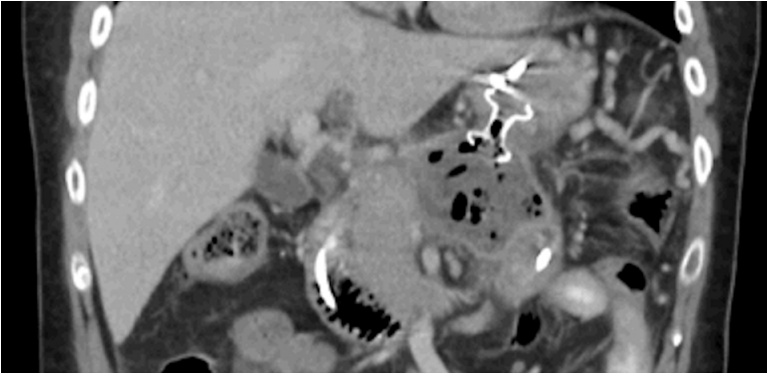
Fig. 4.
Endoscopic view within a walled-off necrosis (WON) cavity accessed with a therapeutic gastroscope through a lumen-apposing metal stents (LAMS). Note the necrotic debris.
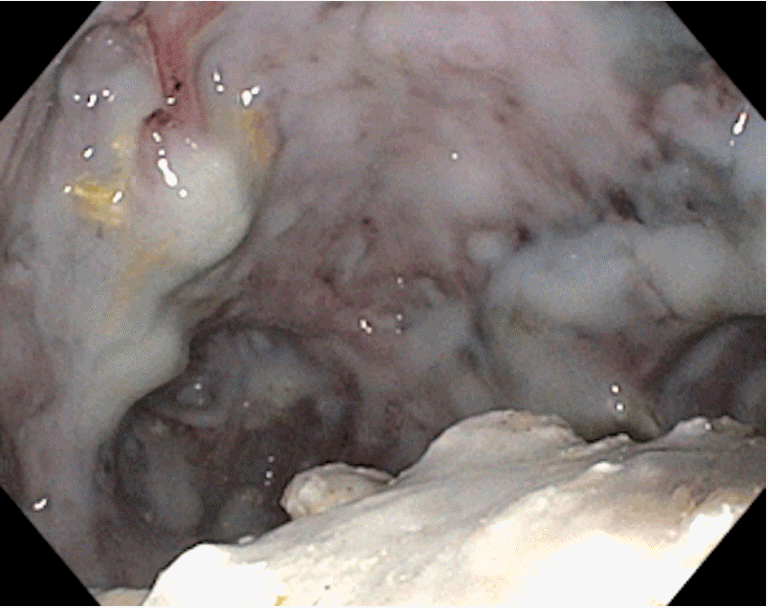
Table 1.
Available Stents for Transmural Drainage of PFCs




 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download