INTRODUCTION
The increasing incidence of rectal carcinoid tumors, also called well-differentiated neuroendocrine tumors (NETs) of the rectum [
1-
3], could be attributed to improved/higher rates of screening colonoscopy, high-resolution enhanced endoscopy, and greater awareness among endoscopists. Most rectal NETs are detected incidentally as they rarely cause carcinoid syndrome. Complete resection is the only definitive curative treatment for rectal NET [
4]. Because of the low risk of metastasis, based on radiological evaluation, rectal NETs measuring <1 cm, as well as selected tumors 1–2 cm in diameter are best treated using endoscopic resection in patients negative for metastasis.
Although complete resection is essential for cure, the complete resection rate of rectal NETs observed with conventional endoscopic mucosal resection (EMR) has been found to range between 52.2% and 84.6% [
5]. Thus, a more effective method of endoscopic resection is necessary. Although endoscopic submucosal dissection (ESD) is noted to achieve high
en bloc and complete resection rates [
6], this procedure is associated with a relatively high complication rate and a long procedure time, as well as the need for a long learning curve to treat rectal NETs. Therefore, in this study, we evaluated the efficacy of precut endoscopic mucosal resection (EMR-P)—a simplified procedure for the treatment of rectal NETs.
Go to :

DISCUSSION
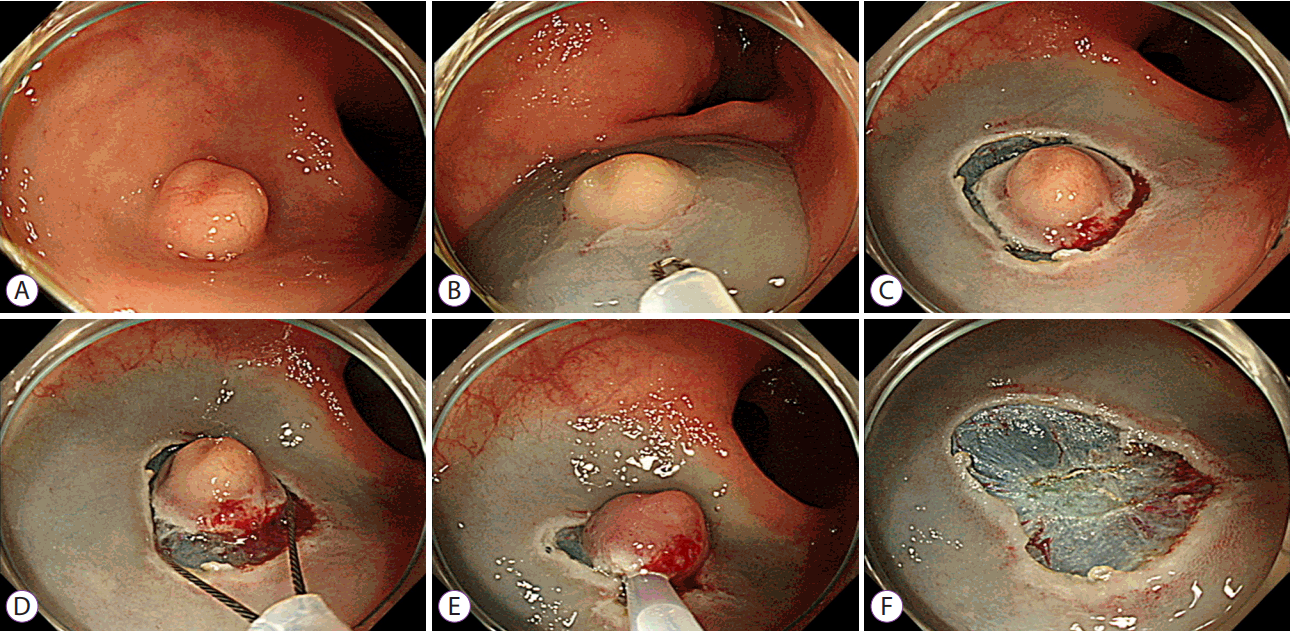
Results from this large case series showed that EMR-P was highly effective for resection of rectal NETs, as shown by the high en bloc and complete resection rates, the acceptable procedure time, and the low complication rate. EMR-P compared well with previous studies that assessed conventional and modified EMR methods for management of rectal NET. The mechanism that explains the efficacy of EMR-P in achieving successful en bloc and complete resection is the method of creating an incision at some distance from the tumor. This increases the likelihood of negative resection margins. Moreover, EMR-P includes positioning and tightening the snare deep in the cut groove, which is created at the outset of the procedure to prevent the snare from slipping while grasping the tumor.
Although most rectal NETs are resected endoscopically, conventional EMR has been showing unsatisfactory complete resection rates, ranging between 52.2% and 84.6% [
5]. There is no clarity regarding the inability of conventional EMR to achieve a high complete resection rate. It could be due in part to the nature of these tumors, which originate from the lower crypts and demonstrate a subepithelial tumor-like growth pattern. Thus, modified EMR techniques have been tried to improve complete resection rates.
Table 4 summarizes outcomes of modified EMR methods used in the resection of rectal NETs in previous studies and our present study. ESD, which can more effectively resect deep-seated tumors, was found to achieve an
en bloc resection rate of 100% and a complete resection rate of 82.6%–100% [
5-
7]. However, despite demonstrating a higher complete resection rate, the procedure time required for ESD is the longest (11.4–24.9 min) among all modified EMR methods. Additionally, ESD necessitates services of an experienced endoscopist, although ESD may be easier to perform for management of rectal NETs compared to the management of large colorectal neoplasms in the proximal colon. We propose that the ESD procedure utilized for the management of rectal NETs should be utilized to train endoscopists intending to perform colorectal ESD [
6]. We suggest that ESD may be examined/assessed as a good treatment option for the management of rectal NETs when ESD specialists or experienced ESD trainees who want to improve their ESD skills are available.
Table 4.
Summary of the Results of Previous Studies of Endoscopic Treatment of Rectal NETs
|
Method |
Study |
No. of patients |
Size (cm)a), mean (SD) |
En bloc resection, (%) |
Complete resection (%) |
Procedure time (min), mean (SD) |
Complicationsb)
|
|
EMR |
Park et al. [6] |
62 |
7.1 (2.3) |
95.2 |
71.0 |
4.2 (3.2) |
Delayed bleeding: 0 (0%) |
|
Perforation: 1 (1.6%) |
|
Lee et al. [7] |
28 |
5.7 (4.0) |
89.3 |
64.3 |
12.0 (12.9) |
None |
|
Kim et al. [10] |
55 |
6.5 (3.2) |
91 |
65.5 |
5.0 (0.8) |
None |
|
Zhao et al. [11] |
10 |
N/A |
80 |
80 |
13.4 (17.13) |
None |
|
Huang et al. [13] |
28 |
9 (2.5) |
96.55 |
82.14 |
4.2 (range, 2–10) |
None |
|
ESD |
Park et al. [6] |
31 |
6.5 (2.6) |
100 |
90.3 |
11.4 (3.7) |
Delayed bleeding: 0 (0%) |
|
Perforation: 1 (3.2%) |
|
Lee et al. [7] |
46 |
6.2 (3.1) |
100 |
82.6 |
18.9 (7.3) |
Delayed bleeding: 0 (0%) |
|
Perforation: 1 (2.2%) |
|
Zhao et al. [11] |
10 |
N/A |
100 |
100 |
24.9 (5.78) |
None |
|
Wang et al. [12] |
25 |
12.27 (3.73) |
100 |
100 |
24.79 (4.89) |
Delayed bleeding: 1 (4.0%) |
|
Perforation: 2 (8.0%) |
|
Cheung et al. [14] |
17 |
7.53 (1.94) |
100 |
88.2 |
20.2 (12.6) |
Delayed bleeding: 0 (0%) |
|
Perforation: 1 (5.9%) |
|
EMR-C |
Zhao et al. [11] |
10 |
N/A |
100 |
100 |
5.2 (0.78) |
None |
|
Wang et al. [12] |
30 |
10.35 (2.95) |
83.3 |
70 |
9.52 (2.14) |
None |
|
EMR-L |
Mashimo et al. [9] |
61 |
6.4 (2.4) |
N/A |
95.2 |
N/A |
Delayed bleeding: 1 (1.6%) |
|
Perforation: 0 (0%) |
|
Kim et al. [10] |
45 |
5.8 (2.4) |
100 |
93.3 |
4.8 (0.9) |
Delayed bleeding: 1 (2.2%) |
|
Perforation: 0 (0%) |
|
EMR-P |
Huang et al. [13] |
31 |
9 (2.5) |
100 |
96.7 |
7.6 (range, 5–13) |
None |
|
Cheung et al. [14] |
16 |
6.63 (1.99) |
87.5 |
81.2 |
9.69 (3.61) |
Delayed bleeding: 0 (0%) |
|
Perforation: 1 (6.3%) |
|
This study |
72 |
6.2 (2.8) |
98.6 |
93.1 |
9.0 (5.6) |
Delayed bleeding: 4 (5.6%) |
|
Perforation: 0 (0%) |

Other modified EMR methods, including cap-assisted EMR and EMR using band ligation, have been suggested as treatment options for rectal NETs [
8]. These methods, while demonstrating complication rates as low as those of conventional EMR, have been shown to achieve higher complete resection rates (70%–100%) compared to conventional EMR (64.3%–82.1%). Their procedure time was acceptable, ranging between 4.8 min and 9.52 min (
Table 4). These methods were as effective and safe as EMR-P, suggesting that any of these modified EMR methods, including EMR-P, can be utilized to resect rectal NETs, and that the selection of procedure is solely at the discretion of the endoscopist.
Our study showed that EMR-P has several advantages over other related procedures: (1) Unlike cap-assisted EMR and EMR using band ligation, which require additional accessories such as cap and ligating devices, respectively, EMR-P does not require any additional instruments because precutting is performed using the snare tip [
9,
10]. (2) Unlike cap-assisted EMR, which is limited in terms of tumor size that can be resected because the tumor must be aspirated through the cap, EMR-P has no such size limitation with respect to tumor resection [
11,
12].
Two previous studies have investigated the usefulness of EMR-P for resection of rectal NET [
13,
14]. These studies, which enrolled 31 and 16 patients, respectively, showed complete resection rates of 96.7%, and 81.2%, respectively, which are comparable to the complete resection rate of 93.1% demonstrated in this present study. These findings indicate that EMR-P is a highly effective endoscopic resection method for the management of rectal NETs. Moreover, EMR-P was found to be safe because no patient demonstrated perforation. Although delayed bleeding did occur, all patients who developed delayed bleeding could be managed endoscopically. Additionally, immediate bleeding was noted to be a predictive factor for the development of delayed bleeding in our present study, as was found in previous reports that describe risk factors for delayed bleeding after EMR of gastric or colorectal polyps [
15-
17]. Thus, it is recommended that endoscopists should pay more attention in patients showing immediate bleeding after EMR-P of rectal NET to reduce the risk of delayed bleeding. Furthermore, the post-resection ulcer can be observed over a longer duration of time such as approximately 5 min to assess and control any visible vessels, which are more likely to be noticed after 5 min than within 1–3 min after colonic EMR [
18].
A strength of our EMR-P method compared to previous studies describing EMR-P [
13,
14] was our use of the snare tip rather than specialized endoknives for precutting. Use of the snare tip reduced the time and cost of this procedure, because additional accessories did not have to be introduced and withdrawn before snaring. To the best of our knowledge, ours is the largest study series evaluating the efficacy of EMR-P for the management of rectal NETs using a single snare device without the need for further knives, which confirms the clinical usefulness of this method in clinical practice.
Limitations of our study: (1) Ours was a retrospective analysis, and selection of the endoscopic resection method used was at the discretion of each endoscopist, which may have resulted in selection bias. However, selection bias was likely not very significant because selection of the endoscopic resection method was not based on any predefined absolute criteria. Additionally, EMR-P was performed in relatively difficult cases, because conventional EMR was generally used when submucosal lifting was satisfactory and secure snaring of the lesion was possible as described previously. Thus, it is possible to infer that EMR-P demonstrated a good performance, based on our study results. (2) Only 51.4% of enrolled patients underwent follow-up endoscopies, and verifying prognosis after EMR-P in this small number of cases who did undergo follow-up endoscopies was difficult. Thus, studies with a greater number of patients are necessary to confirm the accuracy of our follow-up results. (3) The median follow-up period of this study was only 12.6 months. Thus, we could not investigate patients over a long-term follow-up duration to assess local or distant recurrence. However, previous studies describe excellent long-term outcomes after endoscopic resection of rectal NETs ≤10 mm with no recurrence or metastasis noted in any patient after a median duration of 31 months, regardless of resection margin status [
19]. In that study, most patients (107/109) underwent conventional EMR, and the complete resection rate was only 49.5% [
19]. Because our study showed a higher complete resection rate, we reason that prognosis would be excellent, although long-term outcomes were not analyzed directly.
In conclusion, EMR-P using the snare tip for precutting and the same snare for EMR is a highly effective method for resection of rectal NETs, showing high en bloc and complete resection rates, a short procedure time, and an acceptable safety profile without additional costs.
Go to :





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download