INTRODUCTION
Acute renal cortical necrosis (RCN) accounts for approximately 2% of all cases of acute renal failure. RCN has a poor prognosis and usually progresses to chronic renal failure [1,2]. RCN presumably occurs due to small renal blood vessel contraction, but this is unproven. RCN is primarily caused by complications occurring during pregnancy [3]. However, the occurrence of RCN secondary to complications of pregnancy has decreased, while the incidences of non-pregnancy-related causes including viperine snake bite, hemolytic uremic syndrome, renal allograft rejection, acute gastroenteritis, acute pancreatitis, septicemia, and trauma have increased [1,3]. Medications including non-steroidal anti-inflammatory drugs have rarely been reported to cause RCN [3,4].
Here we report a case in which acute RCN developed after tranexamic acid administration to treat gastrointestinal bleeding after an endoscopic papillectomy for an ampullary adenoma.
Go to : 
CASE REPORT
An 82-year-old woman with no significant medical history presented with a 2-month history of abdominal pain and nausea. Vital signs were as follows: blood pressure, 130/80 mm Hg; heart rate, 72 beats/minute (bpm); respiratory rate, 20/minute; and body temperature, 36.4°C. She appeared chronically ill and the findings of a physical examination of the thorax, heart, and abdomen were unremarkable. Laboratory studies revealed a white blood cell (WBC) count of 8,110/μL (neutrophils, 78.5%; and lymphocytes, 17.5%) and a hemoglobin (Hb) of 11.5 g/dL. Serum biochemical values were as follows: blood urea nitrogen (BUN), 12 mg/dL; serum creatinine (Cr), 0.8 mg/dL; aspartate aminotransferase/alanine aminotransferase, 21/14 U/L; albumin, 4.0 g/dL; total bilirubin, 0.6 mg/dL; and amylase 2,460 U/L. Electrolyte levels were as follows: Na, 139 mmol/L; K, 4.0 mmol/L; and Cl, 102 mmol/L. Carbohydrate antigen 19-9 (CA19-9) levels were 37.0 U/mL.
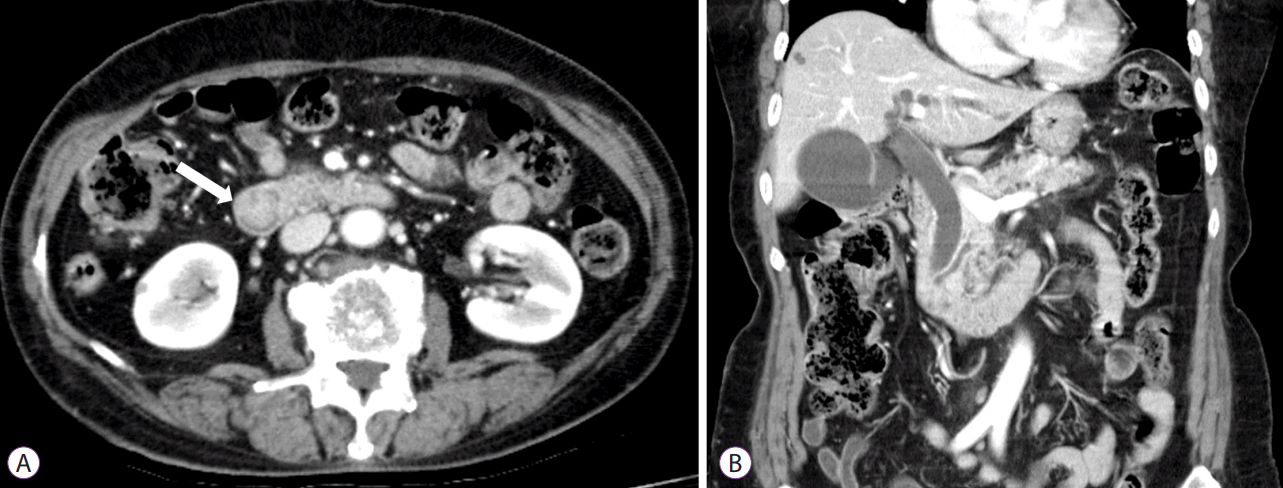
An abdominal computed tomography (CT) scan revealed a 2-cm mass near the ampulla of Vater with mild dilation of the intrahepatic and common bile ducts as well as the pancreatic ducts (Fig. 1). There was no evidence of invasion of other organs. Both kidneys were normal sized without apparent abnormalities.
Endoscopic retrograde cholangiopancreatography with endoscopic ultrasound (EUS) revealed a 2-cm adenoma-like protruding lesion in the ampulla of Vater. EUS showed a well-defined isoechoic homogeneous mass without bile or pancreatic duct invasion (Fig. 2A-C). The mass was resected using a snare and a plastic stent was inserted into the bile duct; insertion of a plastic stent into the pancreatic duct failed. No specific complications, including severe bleeding or perforation, were observed (Fig. 2D). Six hours post-procedure, the patient vomited 50 mL of blood and complained of abdominal pain. A second episode of hematemesis (<30 cc) occurred approximately 5 hours later. Immediately after the second episode, treatment with 1 g of tranexamic acid and 2 KU of hemocoagulase administered three times per day along with ceftriaxone (2 g intravenous) and a proton pump inhibitor for suspected bleeding at the resection site was initiated. Her vital signs were unremarkable. The WBC count was 7,090/μL, Hb was 10.1 g/dL, BUN was 14 mg/dL, Cr was 0.8 mg/dL, and amylase/lipase was 69/54 U/L. After the initial two episodes of hematemesis, no further hematemesis, melena, or hematochezia was observed and the patient’s vital signs remained stable. Her Hb level remained >10 g/dL. Four days after the papillectomy, the patient’s daily urine volume abruptly decreased to <100 cc and she complained of dyspnea. Her blood pressure was 103/86 mm Hg, heart rate was 134 bpm, respiratory rate was 30/minute, and body temperature was 37.0°C. Laboratory studies were as follows: Hb, 9.1 g/dL; amylase/lipase, 415/418 U/L; BUN/Cr, 73/3.9 mg/dL; and brain natriuretic peptide, 5,000 pg/mL. Venous blood gas analysis revealed the following: pH, 6.92: pCO2, 37 mm Hg; and HCO3, 7.6 mmol/L. Pulmonary edema was observed on a chest radiograph and continuous renal replacement therapy (CRRT) was performed to treat metabolic acidosis and pulmonary edema caused by acute renal failure. Although there were no signs of bleeding at that time, 2 units of packed red blood cells were transfused.
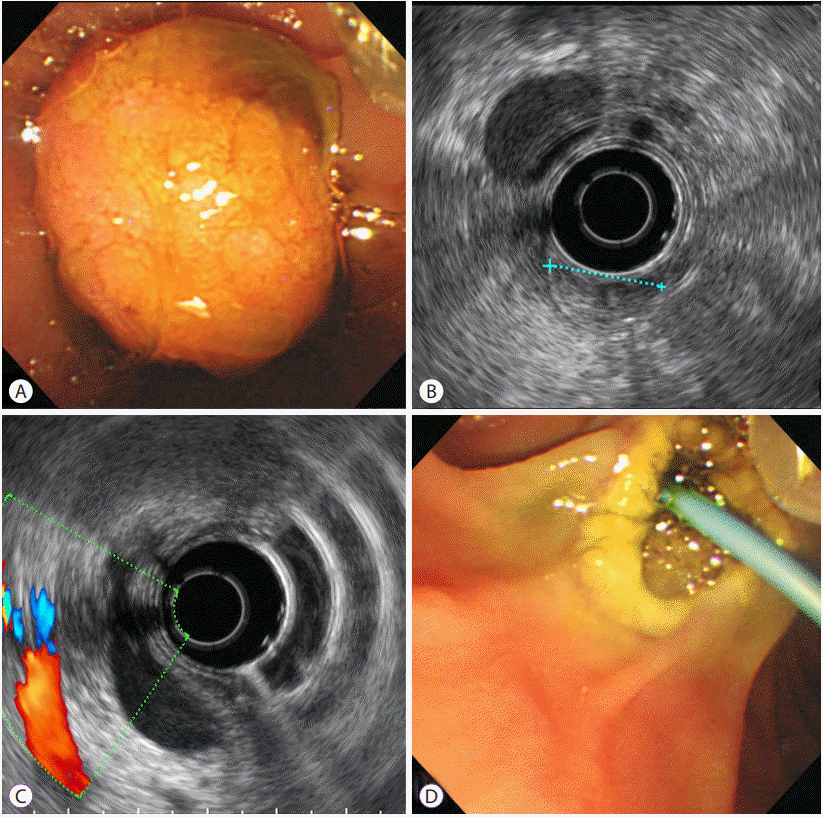 | Fig. 2.A 2-cm adenoma-like protruding lesion was found in the ampulla of Vater on endoscopy (A) and endoscopic ultrasound showed an approximately 17-mm well-defined isoechoic homogeneous mass without bile duct or pancreatic duct invasion. A dilated common bile duct is visible (B, C). After mass resection using a snare and the insertion of a biliary plastic stent, no specific complications, such as severe bleeding or perforation, were observed (D) |
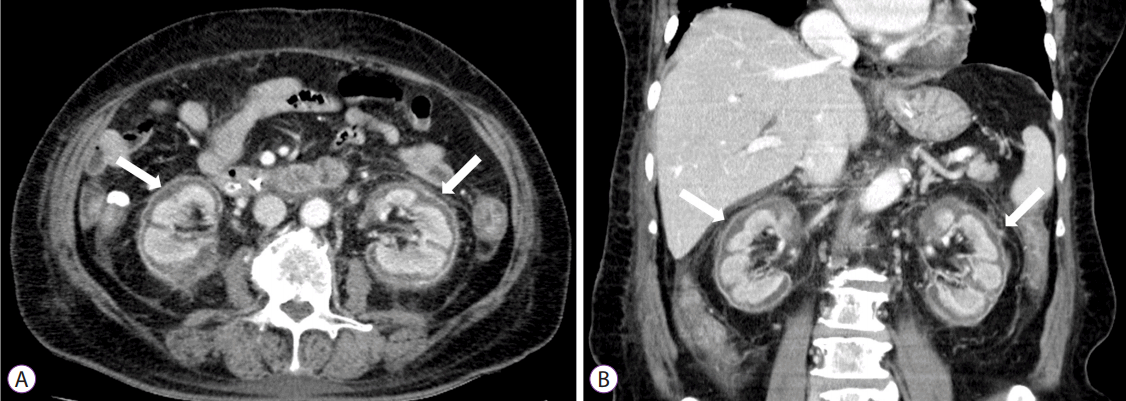
Approximately 10 hours after the CRRT was initiated, the patient was noted to have melena mixed with a small amount of hematochezia. Melena continued to occur 2–3 times/day with a total daily volume of 200–400 cc. Her vital signs were relatively stable: blood pressure, 120–175/65–100 mm Hg; heart rate, 90–120 bpm; respiratory rate, 20–25/minute; and body temperature, 36.0°C–37.8°C. The daily serum Hb level was 7.1–9.5 g/dL and the tranexamic acid with hemocoagulase were continued. The patient received a total of 13.5 g of tranexamic acid and 36 KU of hemocoagulase and received three additional units of packed red blood cells until the sixth day post-procedure, when no further signs of bleeding were observed. However, her daily urine output was nearly zero despite the CRRT. A contrast-enhanced abdominal CT scan was performed on day 12 to assess the cause of her anuric acute renal failure and showed normal-sized kidneys with enhancement of the renal medulla but not of the renal cortex, a finding consistent with acute RCN (Fig. 3). Histopathology revealed a villotubular high-grade adenoma with clear resection margins. Conventional hemodialysis was continued, but renal function did not improve and the oliguria persisted post-discharge. Presently, 6 months after the endoscopic procedure, she continues to undergo hemodialysis. Her most recent BUN/Cr was 22/6.1 mg/dL.
Go to : 
DISCUSSION
Tranexamic acid infusion is a medical treatment for non-variceal upper gastrointestinal bleeding that decreases the need for surgical intervention and mortality. However, tranexamic acid alone is less frequently used than proton pump inhibitors or H2 receptor antagonists in these patients [5]. Tranexamic acid reversibly binds plasminogen during thrombogenesis, interferes with the binding of plasminogen and fibrin, inhibits fibrinolysis, and thus exerts antifibrinolytic effects, promoting thrombus formation and hemostasis. Its side effects include mostly minor gastrointestinal symptoms (e.g., nausea, vomiting, abdominal pain, and diarrhea) and rarely severe complications related to thrombogenesis, such as pulmonary embolism, myocardial infarction, stroke, deep vein thrombosis, and RCN [6,7]. To our knowledge, four cases of tranexamic acid-induced RCN have been reported to date, and ours is the first report of acute RCN in which tranexamic acid was administered for bleeding control after endoscopic papillectomy of an ampullary adenoma [8-11].
In our patient, the volume of the first two episodes of hematemesis was relatively small, and there was no significant decrease in Hb level. Our patient’s vital signs were stable before the development of anuric acute renal failure and dyspnea. Repeat endoscopy was considered because of the patient’s additional bleeding events after CRRT but was not performed because the patient and her family preferred medical treatment alone after a thorough explanation of the risks and benefits of second-look endoscopy for hemostasis of the post-papillectomy bleeding. Fortunately, her bleeding stopped approximately 2 days after CRRT was initiated and 6 days after the endoscopic papillectomy.
Bleeding after endoscopic papillectomy has been reported to occur in 2%–16% of patients, and most episodes can be controlled with conservative management with medical treatment and endoscopic hemostasis [12]. In our patient, the bleeding was successfully controlled by medical treatment and transfusions, but serious acute RCN occurred possibly because of renal artery vasoconstriction caused by tranexamic acid infusion. Hemocoagulase, which causes platelet aggregation at the bleeding site, accelerates fibrin hydrolysis and promotes thrombus formation. It is widely used to manage bleeding in various clinical settings, but no serious complications have been reported [13]. Therefore, RCN was not likely to develop secondary to the administration of hemocoagulase, although the additive thrombotic effect of hemocoagulase may have acted synergistically with tranexamic acid to cause renal artery vasoconstriction.
Although the pathophysiology of RCN is poorly understood, significantly diminished renal arterial perfusion and the release of toxins results in the final common pathway of ischemic necrosis of the renal cortex. Endothelial injury, caused by the potent vasoconstrictor endothelin-1, has been implicated in the pathophysiology of RCN. Vascular endothelial injury can occur through a direct mechanism, as in hemolytic uremic syndrome, eclampsia, and snake bite, or indirectly as in sepsis, pancreatitis, and intravascular hemolysis. The diagnosis of RCN often requires a kidney biopsy, and the histology typically shows tubular cell necrosis with leukocyte infiltration. The glomeruli may be necrotic, and arteriolar thrombosis can be seen with medullary preservation and a thin rim of the subcapsular area. However, invasive biopsy has recently been replaced by non-invasive methods. Hallmark thin cortical lines caused by calcification can be observed on plain film, and a hypoechoic circumferential band in the renal subcapsular area is a characteristic finding on sonography. Contrast-enhanced CT scans are the most sensitive imaging modality, showing bilateral enhancement of the renal medulla and renal subcapsular area with no enhancement of the renal cortex. Renal scans are also useful if contrast-enhanced CT imaging is not available [1,2,14,15].
Acute RCN can be treated with hemodialysis, as in other forms of acute renal failure. However, RCN has a poor prognosis and usually progresses to chronic renal failure. The mortality rate of RCN was 55%−86% prior to 1980 but decreased to 36% after 1980 with improvements in hemodialysis. One-third of RCN patients still require continued hemodialysis [3]. As shown in this rare case of tranexamic acid-induced acute RCN during gastrointestinal bleeding control, clinicians should be aware of the possibility of this serious complication.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download