Abstract
Background/Aims
Peptic ulcer disease (PUD) is a common condition, but is difficult to detect in asymptomatic individuals. We aimed to investigate the prevalence of symptomatic and asymptomatic PUD during screening endoscopy and to identify risk factors for the presence of symptoms in patients with PUD.
Methods
We investigated subjects who underwent a health inspection, including endoscopy of the upper gastrointestinal (GI) tract and a serum anti-Helicobacter pylori IgG assay, and who completed a self-report questionnaire about their symptoms.
Results
Of the 12,852 subjects included in the study, 124 (1.0%) had symptomatic PUD and 309 (2.4%) had asymptomatic PUD. Old age, current smoking, and H. pylori infection were independent risk factors for symptomatic and asymptomatic PUD. Use of non-steroidal anti-inflammatory drugs (NSAIDs) was an independent risk factor only for symptomatic PUD (p=0.040). Compared to subjects with asymptomatic PUD, subjects with symptomatic PUD were more likely to have active-stage ulcers (p=0.002) and to drink more heavily (p=0.005).
Peptic ulcer disease (PUD) is a common disease and results in various complications such as bleeding, perforation, and gastric outlet obstruction [1,2]. Helicobacter pylori infection and the use of non-steroidal anti-inflammatory drugs (NSAIDs) are the most well-known causal factors for PUD [3-7]. Although the prevalence of PUD caused by H. pylori has been decreasing because of eradication therapy, the prevalence of PUD induced by NSAIDs or aspirin is increasing because of the worldwide increase in the aging population [8-10]. In areas of endemic H. pylori infection such as Korea, the prevalence rate of H. pylori infection is as high as 66.9%, and people in this region may be at high risk for PUD. Therefore, new strategies for the prevention and cure of PUD in Korea are important [11].
It is difficult to detect PUD in asymptomatic individuals. In some cases, it is detected because of serious complications, whereas in others, it is detected on screening endoscopy. As the proportion of the population that receives regular health examination increases, the detection of asymptomatic PUD also appears to increase.
According to previous studies, PUD has a strong association with cigarette smoking, advanced age, former alcohol use, obesity, and specific chronic diseases [12]. However, the clinical significance and pathogenic factors associated with asymptomatic PUD remain unclear to date.
Therefore, the present study aimed to investigate the prevalence of symptomatic and asymptomatic PUD in individuals receiving regular medical check-ups in Korea, and we attempted to identify risk factors for the development of symptoms in patients with PUD.
Individuals who underwent a health inspection at the Konkuk University Medical Center between January 2010 and January 2015 were surveyed in this retrospective study. The health inspection included upper gastrointestinal (GI) endoscopy, a serum anti-H. pylori IgG assay, and a self-report questionnaire. The following subjects were excluded from the study: subjects aged <17 years, subjects who underwent GI surgery, subjects with adenoma or carcinoma in the upper GI tract, subjects who did not properly answer the questionnaire, subjects using proton pump inhibitors or histamine-2 receptor antagonists, and subjects previously diagnosed with functional dyspepsia (Fig. 1). In cases in which the result of the serum H. pylori IgG antibody test was equivocal and other H. pylori tests were not performed, the patients were excluded from the study, because the presence of H. pylori infection could not be determined.
Subjects without PUD were categorized into the healthy control group. Among the subjects diagnosed with PUD, subjects with ulcers in the scarring stage were excluded. The remaining subjects were categorized into the symptomatic or asymptomatic PUD group according to the presence of gastroduodenal symptoms.
This study protocol was approved by the Institutional Review Board (IRB) of the Konkuk University School of Medicine (KUH1010767) and was registered in the Clinical Research Information Service (CRIS) ID KCT0001909.
All examinees filled out a self-report questionnaire including questions on gastroduodenal symptoms, smoking, alcohol intake, medical history, underlying disease, and medication history. Pain, soreness, and/or burning sensation localized to the epigastric or upper abdominal area (not substernal area) for at least 3 weeks (at least once each week) during the prior 3 months were considered gastroduodenal symptoms. Drugs taken for more than 1 week during the prior 1 month were reviewed to determine the medication history, including use of NSAIDs, aspirin, proton pump inhibitors, and histamine-2 receptor blockers. Additionally, we determined subjects’ age, sex, weight, and height by reviewing their medical records.
PUD was defined on the basis of the endoscopy findings as a deep mucosal defect with suspected submucosal invasion in the stomach or duodenum in the active or healing stage. Erosive esophagitis was defined as the presence of hyperemic streaks or mucosal breaks in the lower esophagus on endoscopy. The endoscopic procedure was conducted using a standard upper endoscope (GF-260, Olympus, Tokyo, Japan/EPK-I, Pentax, Tokyo, Japan). All subjects provided written consent prior to the procedure.
The H. pylori IgG antibody test was performed using enzyme-linked immunosorbent assay (ELISA) (CHORUS H. pylori IgG, DIESSE Diagnostica Senese, Monteriggioni, Italy) for all included subjects. The test primarily determined the presence of H. pylori infection. A titer of >12 AU/mL was considered to indicate the presence of H. pylori infection, while a titer of <8 AU/mL was regarded to indicate the absence of infection. Titers between 8 AU/mL and 12 AU/mL were regarded as equivocal test findings. If the results of the test were equivocal, we reviewed the patient’s results for other H. pylori tests such as biopsy stained with Giemsa, urea breath test, and rapid urease test. If the result of even one of these tests was positive, the patient was considered to have H. pylori infection. When the H. pylori antibody test result was equivocal and other H. pylori tests were not conducted, the patient in question was excluded from the study. The serum pepsinogen (PG) test measured serum PG I and II concentrations by using a latex particle-enhanced turbidimetric immunoassay (HiBi Co., Anyang, Korea). Corpus atrophy was defined as a serum PG I/II ratio <3.0 and PG I levels <70 ng/mL, as described previously [13].
Continuous variables were analyzed using Student’s t-test, and their values are presented as mean±standard deviation (SD). Categorical variables were analyzed using the χ2 test, and their values are presented as number (%). Logistic regression analyses were performed to estimate the odds ratio (OR) and 95% confidence interval (CI) for factors associated with the existence of PUD, and for factors associated with the presence of symptoms among patients with PUD. All analyses were conducted using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA), and a p-value <0.05 was considered statistically significant.
A total of 12,852 subjects were enrolled in this study, of whom 7,079 (55.1%) were men. Among the total subjects, 12,419 (96.6%) were assigned to the healthy control group, 124 to the symptomatic PUD group (1.0%), and 309 (2.4%) to the asymptomatic PUD group (Table 1). The mean age and body mass index (BMI) of subjects in the healthy control group were 47.65±12.36 years and 23.78±3.32 kg/m2, those of subjects in the symptomatic PUD group were 50.47±11.57 years and 24.30±3.42 kg/m2, and those of subjects in the asymptomatic PUD group were 49.56±12.08 years and 24.47±3.39 kg/m2.
Subjects with symptomatic PUD had higher alcohol consumption than those with asymptomatic PUD (p=0.025) (Table 1). Furthermore, the presence of active-stage ulcers was more common in subjects with symptomatic PUD than in subjects with asymptomatic PUD (p=0.003) (Table 2). Between the symptomatic and asymptomatic PUD groups, there were no significant differences in age, sex, BMI, smoking, H. pylori infection, use of NSAIDs, ulcer location, or ulcer number. Multivariate analysis revealed that heavy drinking (OR, 2.515; 95% CI, 1.315–4.812; p=0.005) and active-stage ulcer (OR, 2.143; 95% CI, 1.323–3.472; p=0.002) were independent risk factors for the presence of symptoms in PUD (Table 3).
Subjects with symptomatic PUD were older (p=0.012), predominantly male (p<0.001), more likely to be smokers (p<0.001), and had higher alcohol consumption (p<0.001) than the healthy control subjects in addition, this group had a higher number of subjects with H. pylori infection (p<0.001), reflux esophagitis (p=0.022), diabetes (p=0.017), and NSAID use (p=0.010) than the healthy control group (Table 1). In contrast, subjects with asymptomatic PUD were older (p=0.007), predominantly male (p<0.001), had a higher BMI (p<0.001), were more likely to be smokers (p<0.001), and had higher alcohol consumption (p<0.001) than the healthy control subjects; this group also had a higher number of subjects with H. pylori infection (p<0.001) and a lower number of subjects with chronic atrophic gastritis (p=0.018) than the healthy control group.
Using multivariate logistic regression analysis, old age (OR, 1.025; 95% CI, 1.008–1.043; p=0.004), current smoking (OR, 3.468; 95% CI, 2.235–5.380; p<0.001), H. pylori infection (OR, 1.899; 95% CI, 1.23–2.917; p=0.003), and use of NSAIDs (OR, 1.905; 95% CI, 1.031–3.519; p=0.040) were found to be independent risk factors for symptomatic PUD (Table 4). Meanwhile, old age (OR, 1.024; 95% CI, 1.013–1.035; p<0.001), male sex (OR, 1.871; 95% CI, 1.362–2.569; p<0.001), current smoking (OR, 2.134; 95% CI, 1.617–2.818; p<0.001), H. pylori infection (OR, 2.186; 95% CI, 1.657–2.883; p<0.001), and absence of atrophic gastritis (OR, 1.854; 95% CI, 1.419–2.424; p<0.001) were independent risk factors for asymptomatic PUD.
In the healthy control group, 1152 (9.3%) subjects had gastroduodenal symptoms (Supplementary Table 1). These subjects were younger (p<0.001), predominantly female (p<0.001), more likely to be smokers (p<0.001), and had higher alcohol consumption (p<0.001) than subjects in the healthy group without gastroduodenal symptoms; in addition, as compared to the healthy group without gastroduodenal symptoms, the healthy group with gastroduodenal symptoms included a lower number of subjects with H. pylori infections (p<0.001), corpus atrophy (p<0.001), and atrophic gastritis (p=0.002), as well as fewer low-dose aspirin users (p=0.033) and a higher number of subjects using NSAIDs (p<0.001).
In the present study, we found that active-stage ulcers triggered more symptoms than healing-stage ulcers, and the presence of active-stage ulcers was an independent risk factor for the presence of symptoms in PUD. Gastroduodenal symptoms can develop more often in the active stage of ulcers, because the gastric mucosa is damaged to the highest extent in this stage. In addition, ulcer location and number were not related to symptoms. It is speculated that patients with healing-stage ulcers show symptoms less frequently, even if they have several peptic ulcers.
In this study, heavy drinking was the most powerful risk factor for the presence of symptoms in patients with PUD. Excessive alcohol consumption by patients with PUD may be related to gastroduodenal symptoms. However, heavy drinking can induce symptoms, with or without PUD [14]. In our study, heavy drinking was related to gastroduodenal symptoms not only in subjects with PUD but also in healthy individuals. Therefore, further studies will be required to determine whether heavy drinking is responsible for the development of symptoms in PUD.
The use of NSAIDs was a risk factor for symptomatic PUD but not for asymptomatic PUD in the present study, and H. pylori infection was a risk factor for both symptomatic and asymptomatic PUD. These findings indicate that NSAID-induced PUD may lead to symptoms more often than H. pylori-associated PUD [15]. According to the current study, the use of NSAIDs was related to gastroduodenal symptoms in the healthy control group as well. Therefore, NSAIDs can cause gastroduodenal symptoms, regardless of the presence of PUD, a finding consistent with previous study results [16].
In the present study, H. pylori infection itself was a powerful risk factor for PUD in Korean adults, regardless of the presence of gastroduodenal symptoms. However, the absence of chronic atrophic gastritis was associated with asymptomatic PUD. This result suggests that ulcer-related symptoms may be reduced in patients with advanced stages of H. pylori infection. As the infection persists over a long period, atrophic changes of the gastric and duodenal mucosa become more severe [17]. However, chronic inflammation of the mucosa may desensitize patients to the mucosal damage and reduce their symptoms [18]. This inverse relationship between gastroduodenal symptoms and chronic atrophic gastritis was also observed in the healthy control group (Supplementary Table 1).
Old age and smoking are well-known risk factors for PUD; these were also found to be risk factors for PUD in the present study, regardless of the presence of symptoms [19]. On the other hand, male sex was a risk factor for asymptomatic PUD, but not for symptomatic PUD in our study. Previous studies revealed that female sex is a common predisposing factor for symptoms of functional GI disorders [20-22]. Because men are less sensitive to GI symptoms, ulcer-related symptoms may be less obvious in men than in women.
Despite our important findings, the present study has some limitations. First, it was nearly impossible to determine the causal relationship between gastroduodenal symptoms and PUD. In this study, 9.3% of subjects in the healthy control group had gastroduodenal symptoms. Although individuals diagnosed with functional dyspepsia were excluded from the study based on survey responses, some subjects included in the study may have had functional GI disorders. Similarly, some symptoms may have been triggered by minor injury to the gastroduodenal wall. In other words, symptoms of some subjects with symptomatic PUD may not be caused by the peptic ulcers. Second, some values were missing in our data, which included the smoking history of 1,614 subjects, the alcohol-consumption history of 640 subjects, and the BMI of 23 subjects; additionally, the serum PG of 7,261 subjects was not measured. Finally, the relationship between atrophic change of the gastric mucosa and upper GI symptoms remains a highly contentious issue [18,23,24]. We believe that severe atrophic change of the gastric mucosa may reduce a patient’s gastroduodenal symptoms, but this hypothesis has not been completely reliable thus far. In the present study, atrophic gastritis diagnosed by endoscopy was inversely related to symptoms. However, PG assay results did not show any relationship to symptoms.
In conclusion, in Korea, a country in which H. pylori infection is endemic, the incidence of symptomatic and asymptomatic PUD found on screening endoscopy was 1.0% and 2.4%, respectively. Old age, current smoking, and H. pylori infection were independent risk factors for symptomatic and asymptomatic PUD. Furthermore, use of NSAIDs was an independent risk factor only for symptomatic PUD. These results suggest that NSAID-induced PUD may lead to symptoms more often than H. pylori-associated PUD. Heavy drinking and active-stage ulcers were independent risk factors for the presence of gastroduodenal symptoms in patients with PUD. Active-stage ulcers may trigger more symptoms than healing-stage ulcers, regardless of their location and number.
Supplementary Material
Supplementary material is available from https://doi.org/10.5946/ce.2016.129 or via http://e-ce.org/.
Supplementary Table 1. Comparison according to the Presence of Symptoms in the Healthy Control Group
REFERENCES
1. Milosavljevic T, Kostić-Milosavljević M, Jovanović I, Krstić M. Complications of peptic ulcer disease. Dig Dis. 2011; 29:491–493.

2. Al Dhahab H, McNabb-Baltar J, Al-Taweel T, Barkun A. State-of-the-art management of acute bleeding peptic ulcer disease. Saudi J Gastroenterol. 2013; 19:195–204.

3. Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999; 340:1888–1899.

4. Hopkins RJ, Girardi LS, Turney EA. Relationship between Helicobacter pylori eradication and reduced duodenal and gastric ulcer recurrence: a review. Gastroenterology. 1996; 110:1244–1252.

5. Atherton JC, Cao P, Peek RM Jr, Tummuru MK, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995; 270:17771–17777.
6. Hunt RH, Yuan Y. Acid-NSAID/aspirin interaction in peptic ulcer disease. Dig Dis. 2011; 29:465–468.

7. Yamaoka Y, Ojo O, Fujimoto S, et al. Helicobacter pylori outer membrane proteins and gastroduodenal disease. Gut. 2006; 55:775–781.

8. Potamitis GS, Axon AT. Helicobacter pylori and Nonmalignant Diseases. Helicobacter. 2015; 20 Suppl 1:26–29.
9. Thorat MA, Cuzick J. Prophylactic use of aspirin: systematic review of harms and approaches to mitigation in the general population. Eur J Epidemiol. 2015; 30:5–18.

10. Sasaki H, Nagahara A, Hojo M, et al. Ten-year trend of the cumulative Helicobacter pylori eradication rate for the ‘Japanese eradication strategy’. Digestion. 2013; 88:272–278.

11. Kim JH, Kim HY, Kim NY, et al. Seroepidemiological study of Helicobacter pylori infection in asymptomatic people in South Korea. J Gastroenterol Hepatol. 2001; 16:969–975.

12. Lau JY, Sung J, Hill C, Henderson C, Howden CW, Metz DC. Systematic review of the epidemiology of complicated peptic ulcer disease: incidence, recurrence, risk factors and mortality. Digestion. 2011; 84:102–113.

13. Choi HS, Lee SY, Kim JH, et al. Combining the serum pepsinogen level and Helicobacter pylori antibody test for predicting the histology of gastric neoplasm. J Dig Dis. 2014; 15:293–298.
14. Halder SL, Locke GR 3rd, Schleck CD, Zinsmeister AR, Talley NJ. Influence of alcohol consumption on IBS and dyspepsia. Neurogastroenterol Motil. 2006; 18:1001–1008.

15. Kim HM, Cho JH, Choi JY, et al. NSAID is inversely associated with asymptomatic gastric ulcer: local health examination data from the Korean National Health Insurance Corporation. Scand J Gastroenterol. 2013; 48:1371–1376.

16. Yap PR, Goh KL. Non-steroidal anti-inflammatory drugs (NSAIDs) induced dyspepsia. Curr Pharm Des. 2015; 21:5073–5081.

17. D’Elios MM, Andersen LP. Inflammation, immunity, and vaccines for Helicobacter pylori. Helicobacter. 2009; 14 Suppl 1:21–28.
18. Tahara T, Arisawa T, Shibata T, et al. Association of endoscopic appearances with dyspeptic symptoms. J Gastroenterol. 2008; 43:208–215.

19. Li LF, Chan RL, Lu L, et al. Cigarette smoking and gastrointestinal diseases: the causal relationship and underlying molecular mechanisms (review). Int J Mol Med. 2014; 34:372–380.

20. Lee SP, Sung IK, Kim JH, Lee SY, Park HS, Shim CS. The effect of emotional stress and depression on the prevalence of digestive diseases. J Neurogastroenterol Motil. 2015; 21:273–282.

21. Ford AC, Bercik P, Morgan DG, Bolino C, Pintos-Sanchez MI, Moayyedi P. Characteristics of functional bowel disorder patients: a cross-sectional survey using the Rome III criteria. Aliment Pharmacol Ther. 2014; 39:312–321.

22. Oshima T, Miwa H. Epidemiology of functional gastrointestinal disorders in Japan and in the world. J Neurogastroenterol Motil. 2015; 21:320–329.

Fig. 1.
Flow chart of inclusion and exclusion of subjects. A total of 16,008 people who underwent a health inspection (gastroscopy, a self-report questionnaire, and a serum Helicobacter pylori IgG antibody test) at our center were investigated. Subjects younger than 17 years of age, subjects who underwent upper gastrointestinal (GI) surgery, subjects with adenoma or carcinoma in the upper GI tract, subjects who did not properly answer the questionnaire, subjects using proton pump inhibitors or histamine-2 receptor antagonists, and subjects previously diagnosed with functional dyspepsia were excluded from the study. If the results of the serum H. pylori IgG antibody test were equivocal and other H. pylori tests were not performed, subjects were excluded, because the presence of H. pylori infection could not be determined. Subjects without peptic ulcer disease (PUD) were categorized into the healthy control group. Among subjects diagnosed with PUD, subjects with ulcers in the scarring stage were excluded. The remaining subjects were categorized into the symptomatic or asymptomatic PUD group according to the presence of symptoms.
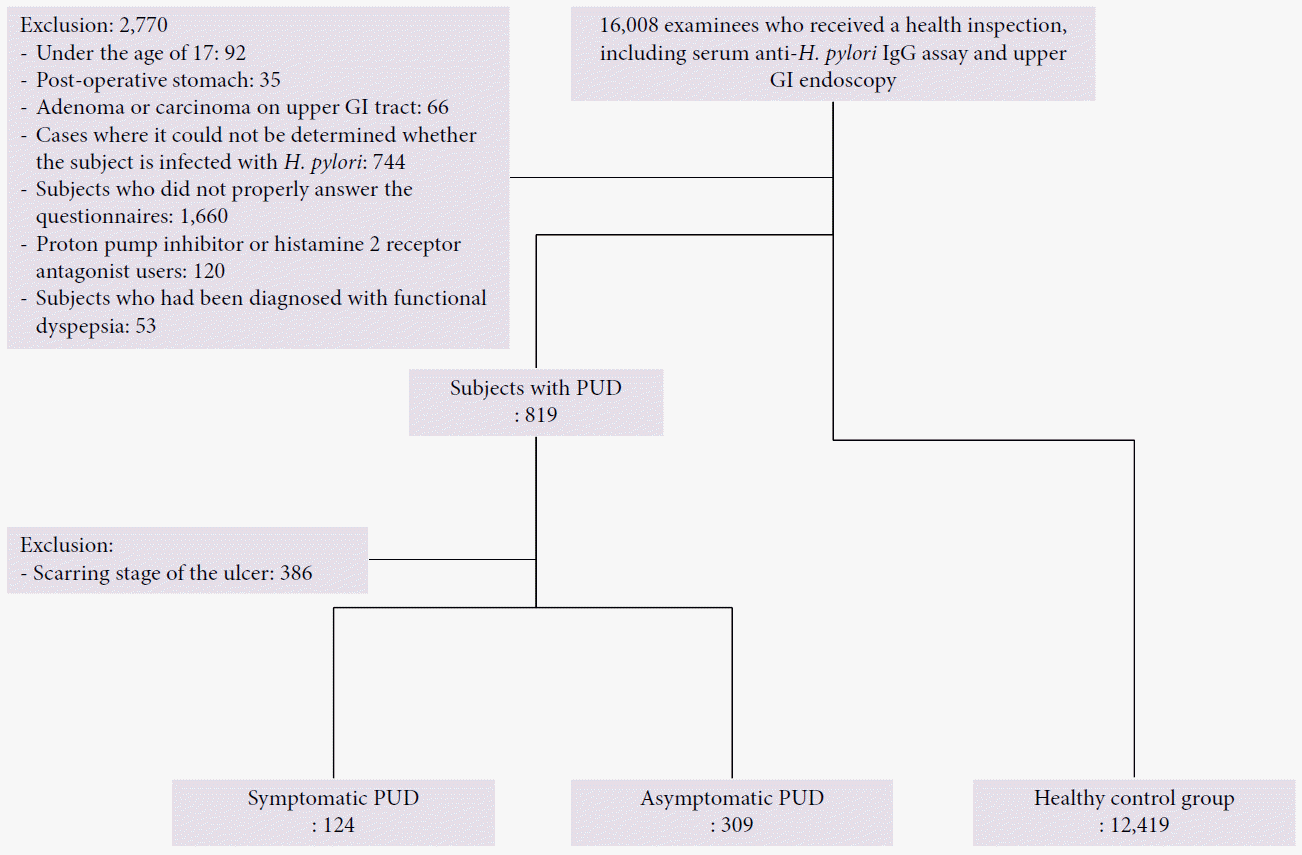
Table 1.
Baseline Characteristics of All Subjects and Comparison among the Groups
| Variables | Healthy control group (A, n=12,419) | Symptomatic PUD (B, n=124) | Asymptomatic PUD (C, n=309) | p-value (A vs. B) | p-value (A vs. C) | p-value (B vs. C) |
|---|---|---|---|---|---|---|
| Age, yearsa) | 47.65 (±12.36) | 50.47 (±11.57) | 49.56 (±12.08) | 0.012 | 0.007 | 0.476 |
| Male sex, n (%) | 6758 (54.4) | 94 (75.8) | 227 (73.5) | <0.001 | <0.001 | 0.630 |
| BMI, kg/m2 a) | 23.78 (±3.32) | 24.30 (±3.42) | 24.47 (±3.39) | 0.082 | <0.001 | 0.627 |
| Smoking, n (%) | <0.001 | <0.001 | 0.224 | |||
| Non-smoker | 5871 (54.1) | 28 (26.2) | 85 (31.1) | |||
| Past smoker | 2705 (24.9) | 25 (23.4) | 77 (28.2) | |||
| Current smoker | 2282 (21.0) | 54 (50.5) | 111 (40.7) | |||
| Alcohol, n (%) | <0.001 | <0.001 | 0.025 | |||
| Non-drinker | 2980 (25.3) | 14 (11.7) | 42 (14.2) | |||
| Social drinker | 8208 (69.6) | 86 (71.7) | 230 (78.0) | |||
| Heavy drinkerb) | 609 (5.2) | 20 (16.7) | 23 (7.8) | |||
| H. pylori infection, n (%)c) | 6865 (55.3) | 89 (71.8) | 218 (70.6) | <0.001 | <0.001 | 0.816 |
| Corpus atrophy, n (%)d) | 721 (13.4) | 5 (7.4) | 13 (8.7) | 0.155 | 0.111 | 0.798 |
| Endoscopic findings, n (%) | ||||||
| Chronic atrophic gastritis | 5646 (45.5) | 47 (37.9) | 119 (38.5) | 0.103 | 0.018 | 0.914 |
| Reflux esophagitis | 984 (7.9) | 17 (13.7) | 26 (8.4) | 0.022 | 0.831 | 0.110 |
| Underlying diseases, n (%) | ||||||
| Diabetes | 799 (6.4) | 15 (12.1) | 24 (7.8) | 0.017 | 0.348 | 0.193 |
| Medication, n (%) | ||||||
| NSAIDs | 765 (6.2) | 15 (12.1) | 27 (8.7) | 0.010 | 0.073 | 0.369 |
| Low dose aspirin | 845 (6.8) | 7 (5.6) | 28 (9.1) | 0.722 | 0.137 | 0.253 |
a) Continuous variables were summarized as mean±standard deviation (SD) and analyzed by the student t-test. All other data were presented as number (%) and analyzed by the chi-square test. The significant results are highlighted in bold.
b) Heavy drinking was defined as consuming 15 drinks or more per week for men and 8 drinks or more per week for women.
Table 2.
Characteristics of the Peptic Ulcer
Table 3.
Risk Factors for the Presence of Symptoms in PUD
Table 4.
Risk Factors for Symptomatic and Asymptomatic PUD




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download