INTRODUCTION
Endoscopic resection is regarded as the standard treatment for gastric neoplasms because of its minimal invasiveness and favorable outcomes [
1,
2]. With effective endoscopic surveillance, gastric neoplasms are increasingly diagnosed at an early stage and are possible candidates for endoscopic resection [
3].
Endoscopic examination with biopsy is the gold standard for the diagnosis of gastric neoplasm. However, neoplastic lesions can be missed during endoscopic examination [
4,
5]. In patients with previously diagnosed gastric neoplasm, if the information from the referring center is insufficient, or a definite target for treatment needs to be identified, repeat endoscopy is performed before deciding on the treatment strategy. Moreover, during endoscopic resection, endoscopists occasionally detect an additional lesion that was not diagnosed in previous examinations, and this may lead to unscheduled procedures or modification of the therapeutic plan. Accurate detection of multifocal lesions before endoscopic resection is, therefore, necessary for the successful management of gastric neoplasms.
The prevalence of synchronous lesions after endoscopic resection has been reported to be 2.0% to 11.6% [
6-
8]. The majority of studies reporting the prevalence and diagnosis of synchronous gastric neoplasms have focused on the prevalence after previous endoscopic examinations that were performed within a certain period of time [
6,
8-
10]. Recently, one such study suggested that one-third of those lesions could have been detected during the previous endoscopic examinations [
7]. We investigated the characteristics of simultaneous lesions missed before endoscopic resection for gastric neoplasm, aimed primarily at elucidating the reason for missed diagnoses.
MATERIALS AND METHODS
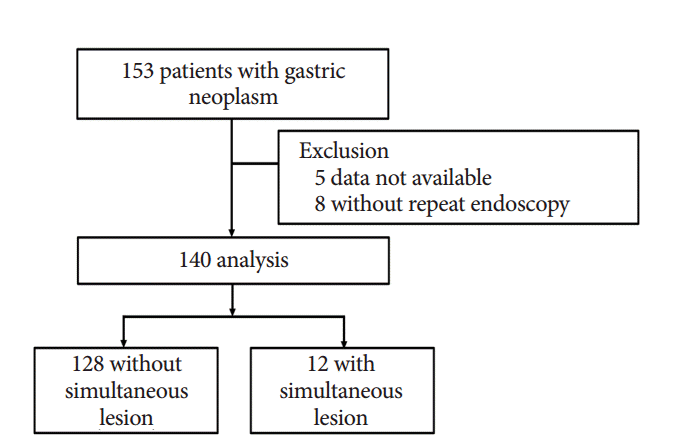
Patients referred to Asan Medical Center for further evaluation of gastric neoplasm and who underwent repeat endoscopy between June 2013 and June 2014 were eligible for this study. During the study period, a total of 153 patients with gastric neoplasms were consecutively referred to a single physician (JHL) at our center. Of these patients, five whose medical records and endoscopic images were missing and eight who did not undergo repeat endoscopy were excluded; the remaining 140 cases were analyzed (
Fig. 1). The study protocol was approved by the Institutional Review Board of Asan Medical Center.
Repeat esophagogastroduodenoscopy was performed before endoscopic resection to confirm the location of the index neoplasm and to investigate the presence of any additional lesions. Endoscopic examinations were performed using a CV-260SL system (Olympus, Tokyo, Japan) with a high-resolution GIF-H260 or GIF-H260Z endoscope (Olympus). In addition to conventional white-light endoscopy, narrow band imaging or chromoendoscopy was used during the examination to further characterize the lesions. Biopsy specimens were obtained from all suspicious lesions for histological confirmation.
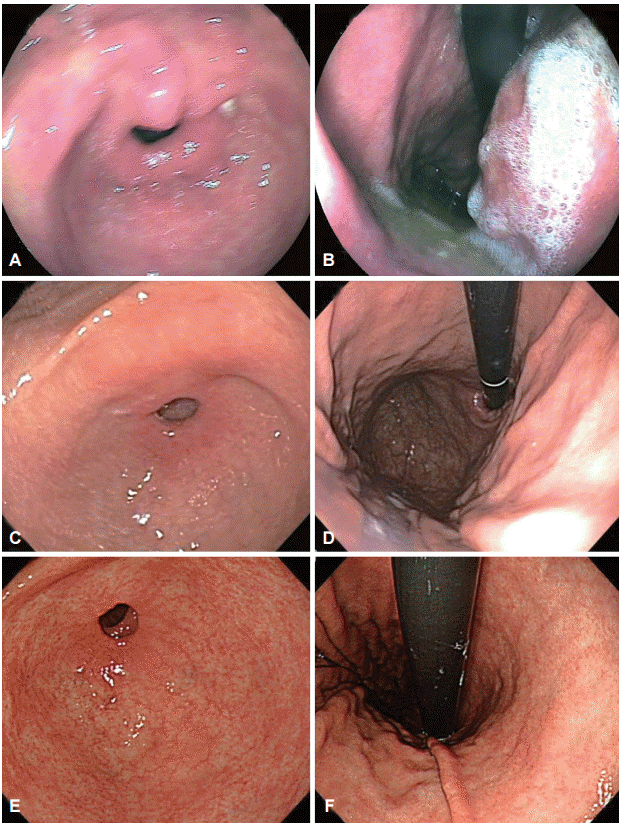
Clinicopathologic features, including tumor factors and procedure-related factors, were retrospectively reviewed from medical records. We evaluated the endoscopic images taken at the referring center and classified the image quality as poor, fair, or good (
Fig. 2). If there was a blurring of images or disturbances such as gastric content or unwashed mucus, the image was considered poor. If the gastric mucosa was clear and well-visualized, the image was considered good. If most images could be interpreted but some portion of the gastric mucosa was not clearly visualized, the image was considered fair. Valid images were defined as images of an area other than the primary lesion that had previously been diagnosed as gastric neoplasm. The total number of images of the scanned gastric mucosa and the number of valid images were counted.
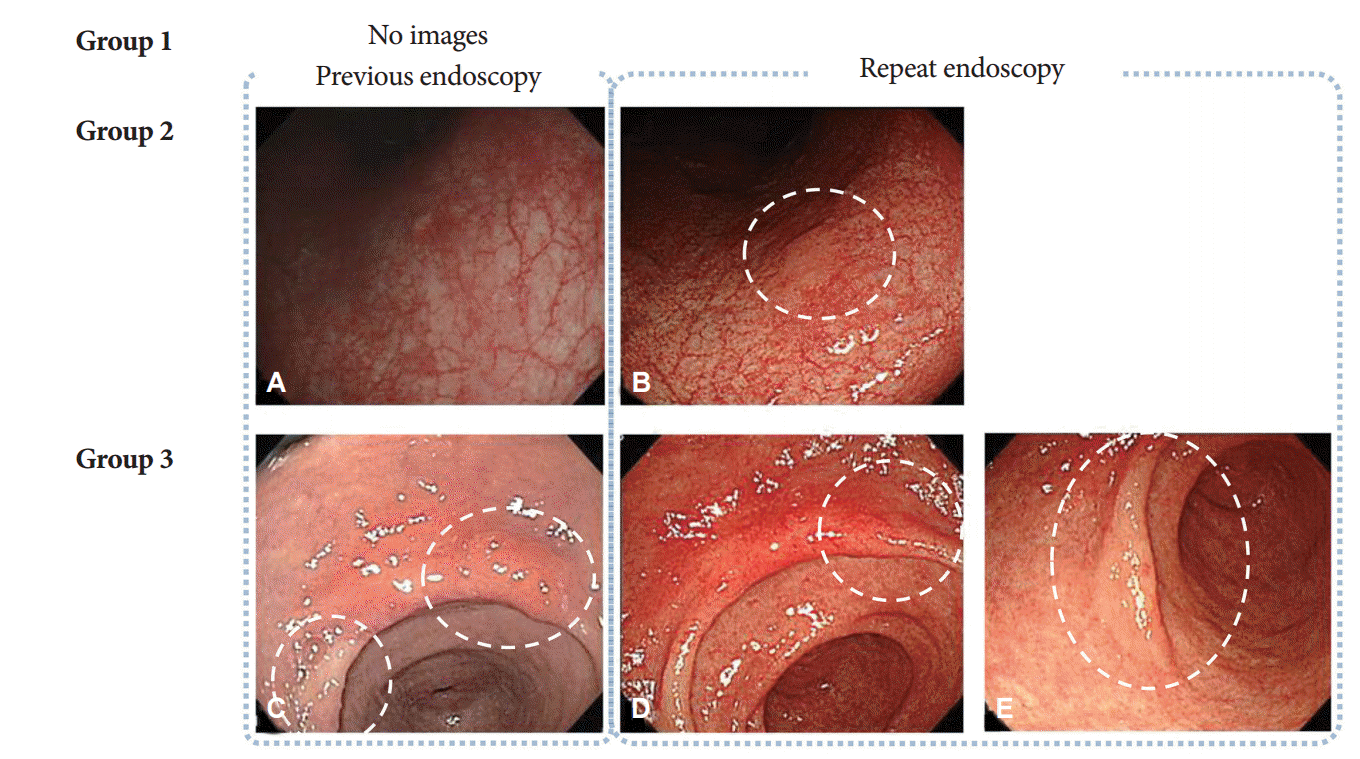
Simultaneous lesions were defined as lesions detected for the first time on repeat endoscopy. They were classified into three groups based on a comparison of the endoscopic images obtained in the earlier and repeat examinations: group 1, no available previous image of the area where the simultaneous lesion was detected; group 2, an image of the area where the simultaneous lesion was located was available but the corresponding lesion was not clearly visible in the earlier images; and group 3, the simultaneous lesion was visible in the earlier images but no biopsy was performed at the time. Representative cases from groups 2 and 3 are shown in
Fig. 3. The descriptions of gastric neoplasm, including the location and macroscopic types, were based on the classification proposed by the Japanese Gastric Cancer Association [
11].
Statistical analysis
Differences between clinical characteristics were determined using Student t-test, the chi-square test, Mann-Whitney U-test, or Fisher exact test as appropriate. The paired t-test or Wilcoxon signed-rank test was used to analyze paired data. SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses, and a p<0.05 was considered significant.
DISCUSSION
In the present study, we investigated the characteristics of missed simultaneous lesions before endoscopic resection for gastric neoplasm, with the primary aim of clarifying the reason for missed diagnoses. In the population studies, simultaneous lesions were detected in 8.6% of patients, and lesions disregarded or unnoticed during the original examination were the major reasons for missed diagnosis. Procedure-related factors such as procedure time did not differ in patients with and without simultaneous lesions.
Endoscopic resection of gastric neoplasm leaves most of the normal gastric mucosa intact and further neoplasms can be present or arise in the remaining gastric mucosa. Additional treatment such as repeated endoscopic resection or gastrectomy for missed gastric neoplasm will result in increased cost, and therefore, efforts should be made to detect simultaneous lesions before endoscopic resection once a gastric neoplasm has been diagnosed. The prevalence of missed synchronous lesions has been variously reported, and several factors including pathologic diagnosis of the primary lesion (adenoma), the number of biopsies taken, and small lesion size have been recognized as risk factors for failure to detect additional lesions during endoscopy [
5-
7,
13,
14]. In those studies, a synchronous lesion was defined as a lesion detected within 1 year after the index examination or endoscopic resection. We hypothesized that some of the lesions may have been present at the time of the initial diagnosis, and we therefore, investigated the prevalence of simultaneous lesions prior to the treatment. We found simultaneous lesions in 8.6% of patients before endoscopic resection; these lesions may have led to additional sessions of treatment if they had been disregarded or missed.
Failure to detect simultaneous lesions may be due to several reasons as follows: the lesions are not seen, are seen but not biopsied, are biopsied inadequately, or are interpreted incorrectly by the pathologist [
4,
7,
10]. It is noteworthy that almost all missed diagnoses are due to errors by endoscopists. Occasionally, the procedure is performed hastily, without thoroughness, and overlooking the purpose of endoscopy. In the present study, more than half of the cases with missed diagnoses had images of the simultaneous lesion documented in the previous endoscopic examination but they were not biopsied (group 3). Since most of the simultaneous lesions were located in the lower third of the stomach, some of them may have been missed because the endoscopist was concentrating on evaluating the primary lesion or because the evaluation was limited. On the other hand, in more than one-third of the cases there was no documented image of the particular location where the simultaneous lesion was detected (group 1). The majority of these lesions were located in the anterior or posterior wall of the stomach, which is consistent with a report suggesting that the posterior wall of the body is a blind spot [
6]. Endoscopists should always consider the possibility of multifocal or synchronous lesions and should attempt to examine the entire gastric mucosa.
There was one case classified as group 2, which was defined as a simultaneous lesion that was not evident in the earlier images. Group 2 lesions may have been missed because the endoscopist did not recognize the early signs of dysplastic changes, or because the lesion had developed after the index examination. Initially, there may have been only a subtle change that was hardly noticeable at the time of the previous examination. In addition, atrophic or metaplastic changes of the background mucosa may have been a reason for the missed diagnosis. In one study, even advanced gastric cancers were definitely missed and endoscopy performed 3 or 4 months before the final diagnosis did not reveal any abnormalities, supporting the possibility of missed diagnosis rather than rapidly growing cancer [
10]. Meticulous endoscopic examination is important, and additional techniques such as narrow band imaging or chromoendoscopy might be useful for enhancing diagnostic performance [
15-
17].
Although the importance of implementing a process for maintaining and enhancing the quality of endoscopy has been emphasized in clinical practice, there have been no clearly documented quality indicators regarding esophagogastroduodenoscopy. Recently proposed quality indicators include measures of process and outcome, and recommend photodocumentation of important anatomic landmarks and pathologic findings during endoscopic examination [
18]. Endoscopic images reflect the quality and completeness of endoscopic evaluation and provide information about pathology that facilitates consultation with other physicians. Although the evidence is limited, it may be worthwhile to consider repeat endoscopy to ultimately reach treatment decisions, particularly if previously obtained information is suboptimal or equivocal.
The recommendations also emphasize that the number of procedures performed in training should not be used to define competence, but that objective measurement of performance is essential [
18]. According to one report, procedure times of more than 10 minutes were more frequent when a simultaneous lesion was detected, and the probability of detecting synchronous neoplasms decreased by 6.9% as the duration of endoscopic examination decreased by 1 minute [
7]. However, in our study neither the duration of endoscopic examination nor the absolute differences in the procedure times between previous and repeat procedures were significantly different. Hence, prolonging a procedure does not always guarantee a higher quality of endoscopic examination. A more appropriate quality indicator which can reflect the completeness of the examination is needed.
Our study has several limitations. First, because our analyses were retrospective and based on a database from a single physician, selection bias and referral bias cannot be excluded. Second, we could not evaluate operator-dependent factors, including the level of experience, and this might have influenced the detection rate. In addition, the duration of endoscopic examination was shorter than that of a previous study [
7], and the possibility of missed diagnosis even after several sessions of repeat endoscopy could not be excluded. Third, we could not calculate the cost-effectiveness of repeated endoscopic examination. Although the detection of simultaneous gastric neoplasms before endoscopic resection enables the treatment of all the lesions at a single time, the cost and benefit of such treatment is uncertain. The cost and risk of repeat endoscopy with biopsy may outweigh the potential benefits of additional information about the primary lesion, which only rarely modifies the treatment strategy [
19]. In addition, not only incorrect endoscopic resection but also the presence of simultaneous lesions contribute to the increase in cost. In our study, three cases of early gastric cancers, including undifferentiated cancers located in the upper third of the stomach, were detected by repeat endoscopy and may have led to a change in treatment strategy. Another limitation is that a relatively small number of patients were included in the analysis. The prevalence of simultaneous lesions was 8.6% in our study, and this may have been an underestimate.
In conclusion, lesions disregarded or unnoticed during the initial endoscopic examination were the main reason for missed diagnosis of simultaneous lesions. Meticulous and complete evaluation of the entire gastric mucosa, rather than of specific key areas, is mandatory for detecting both the index lesion and any simultaneous lesions. Repeat endoscopy may provide additional information before definitive treatment regardless of the quality of the previously obtained images.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download