INTRODUCTION
MATERIALS AND METHODS
General indications for CMI-EMR and ESD
Endoscopic submucosal dissection
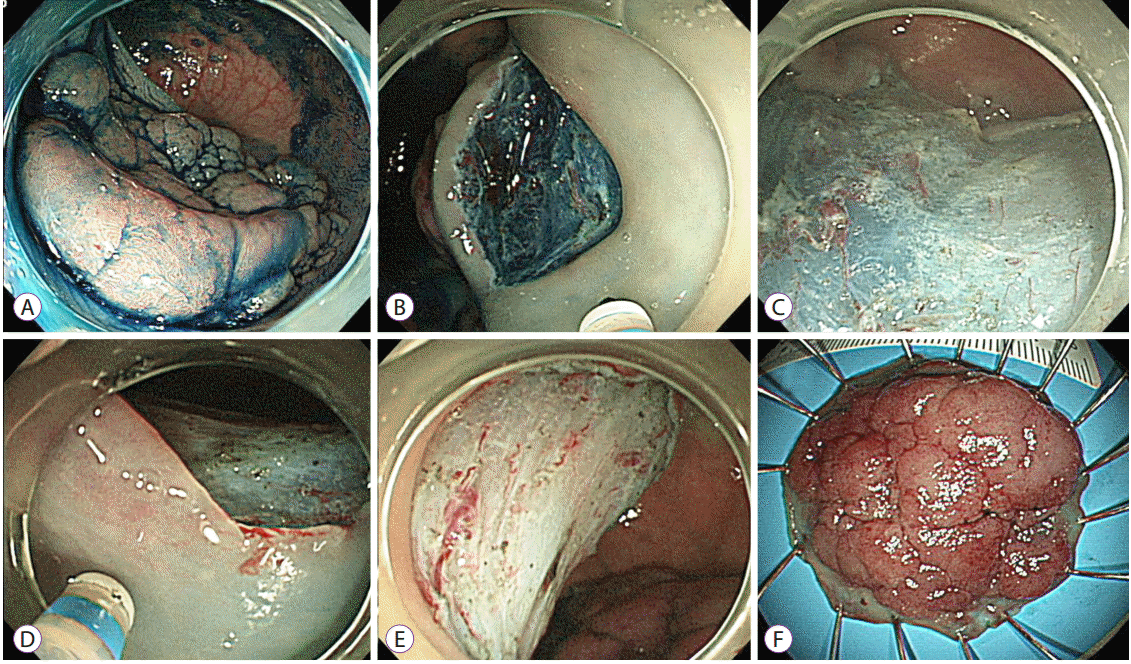 | Fig. 1.Representative example of an endoscopic submucosal dissection. (A) A laterally spreading tumor on the rectum. (B) The submucosal layer was exposed after precutting and trimming. (C) An additional submucosal dissection was performed. (D) After dissecting more than 75% of the lesion, a mucosal incision was made on the oral side. The scope was retroflexed in this image. (E) A clean-based artificial ulcer remained after complete excision of the lesion. (F) The lesion was removed en bloc. |
Endoscopic mucosal resection with circumferential mucosal incision
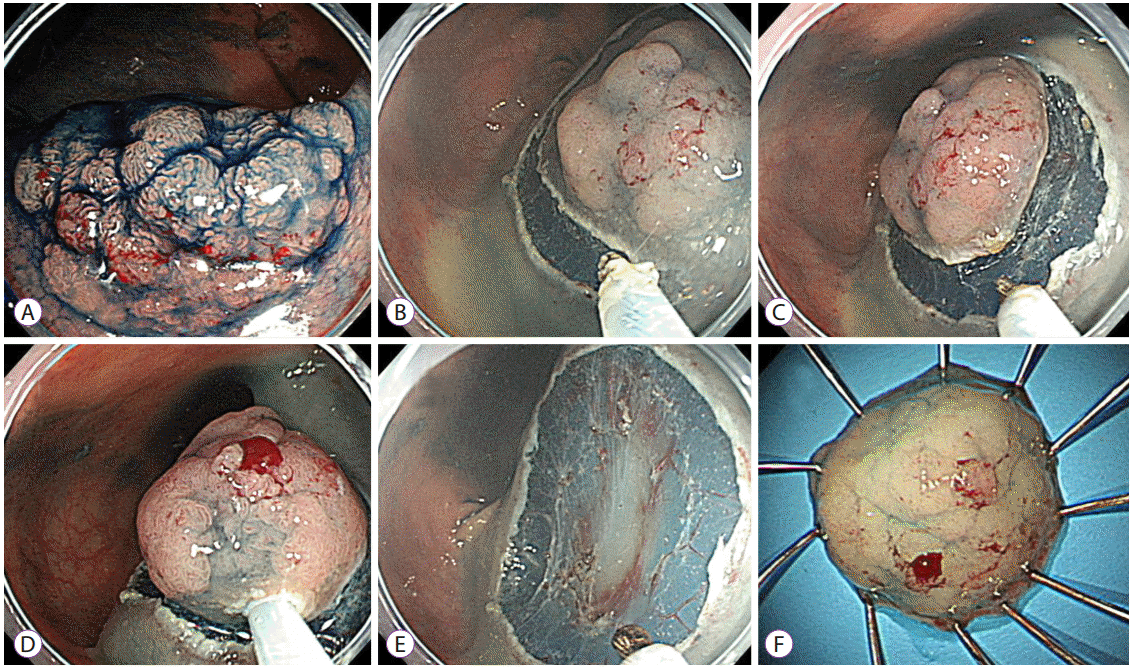 | Fig. 2.Representative example of an endoscopic mucosal resection with circumferential mucosal incision. (A) A 20-mm-sized laterally spreading tumor was noted on the rectum. (B, C) After submucosal injection using sodium hyaluronate solution, a circumferential mucosal incision was performed using the tip of the snare. (D, E) The lesion was snared along with the circumferential groove and resected en bloc. (F) The lesion was identified as a villotubular adenoma with a high-grade dysplasia of 22×18 mm in size. |
Pre-medication and procedure-related patient care
Specimen histology
Patient selection
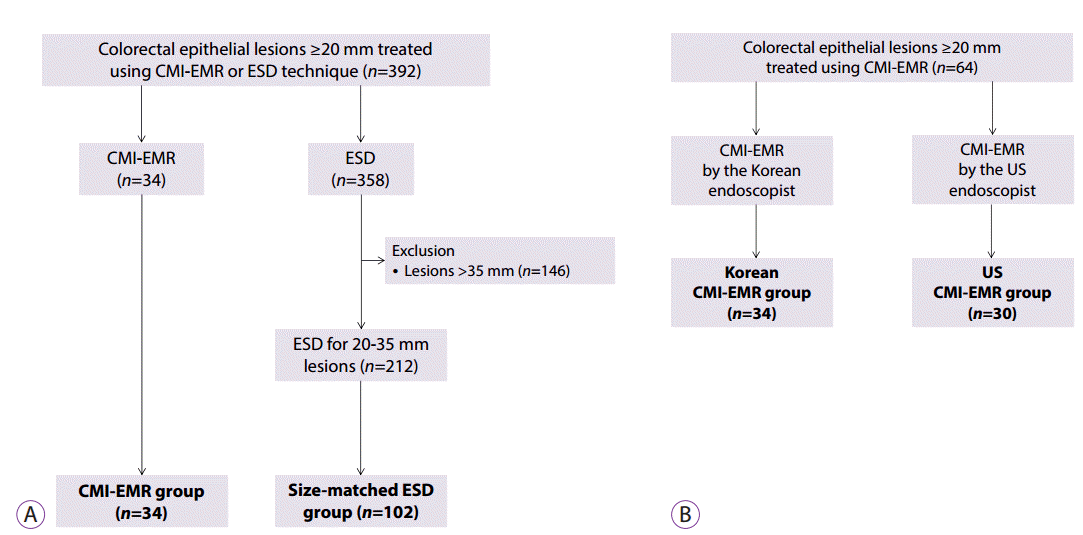 | Fig. 3.Selection of cases. (A) Case selection from among the ≥20-mm colorectal lesions that were removed using endoscopic mucosal resection with circumferential mucosal incision (CMI-EMR) or endoscopic submucosal dissection (ESD) at a Korean center. The size of the lesions removed using CMI-EMR ranged between 20 and 35 mm. After excluding lesions larger than 35 mm, size-matched ESD cases were randomly selected and matched with the CMI-EMR cases at a 1:3 ratio. (B) CMI-EMR cases performed by an experienced Korean endoscopist and an inexperienced American endoscopist were categorized as the Korean CMI-EMR group and the US CMI-EMR group, respectively. |
Data collection
Statistical analysis
RESULTS
Comparison between the CMI-EMR and ESD cases treated by a Korean endoscopist
Table 1.
| Variables | CMI-EMR group (34 lesions in 34 patients) | Size-matched ESD group (102 lesions in 100 patientsa)) | p-value |
|---|---|---|---|
| Age, yr, mean±SD | 61.6±8.0 | 62.2±10.1 | 0.730 |
| Sex, male, n (%) | 18 (52.9) | 61 (59.8) | 0.482 |
| Antiplatelet agentsb), n (%) | 1 (2.9) | 1 (1.0) | 0.439 |
| Coagulopathy, n (%) | 0 | 0 | NA |
| Thrombocytopeniac), n (%) | 1 (2.9) | 0 | 0.250 |
| Size, mm, mean±SD | 22.3±3.9 | 22.9±2.4 | 0.268 |
| Locationd), n (%) | 0.289 | ||
| Right colon | 19 (55.9) | 45 (44.1) | |
| Left colon | 8 (23.5) | 21 (20.6) | |
| Rectum | 7 (20.6) | 36 (35.3) | |
| Morphology, n (%) | 0.999 | ||
| Is | 4 (11.8) | 13 (12.7) | |
| II (IIa, IIb, or IIa + IIc) | 30 (88.2) | 89 (87.3) | |
| Histology, n (%) | 0.258 | ||
| TA, TVA, or VA | 28 (82.4) | 81 (79.4) | |
| SSA/P | 5 (14.7) | 6 (5.9) | |
| Superficial submucosal cancer | 1 (2.9) | 8 (7.8) | |
| Deep submucosal cancer | 0 | 6 (5.9) | |
| Non-neoplastic lesions | 0 | 1 (1.0) |
Table 2.
Comparison between the Korean CMI-EMR and US CMI-EMR groups
Table 3.
| Variables | Korean CMI-EMR group (n=34) | US CMI-EMR group (n=30) | p-value |
|---|---|---|---|
| Age, yr, mean±SD | 61.6±8.0 | 67.6±7.9 | 0.004 |
| Sex, male, n (%) | 18 (52.9) | 21 (70) | 0.163 |
| Antiplatelet agentsa), n (%) | 1 (2.9) | 7 (23.3) | 0.021 |
| Coagulopathy, n (%) | 0 | 0 | NA |
| Thrombocytopeniab), n (%) | 1 (2.9) | 0 | 0.999 |
| Size, mm, mean±SD | 22.3±3.4 | 23.2±4.7 | 0.405 |
| Locationc), n (%) | 0.331 | ||
| Right colon | 19 (55.9) | 21 (70) | |
| Left colon | 8 (23.5) | 3 (10) | |
| Rectum | 7 (20.6) | 6 (20) | |
| Morphology, n (%) | 0.495 | ||
| Is | 4 (11.8) | 6 (20) | |
| II (IIa, IIb, or IIa + IIc) | 30 (88.2) | 24 (80) | |
| Histology, n (%) | 0.604 | ||
| TA, TVA, or VA | 28 (82.4) | 21 (70) | |
| SSA/P | 5 (14.7) | 8 (26.7) | |
| Superficial submucosal cancer | 1 (2.9) | 1 (3.3) |
Table 4.
| Variables | Korean CMI-EMR group (n=34) | US CMI-EMR group (n=30) | p-value |
|---|---|---|---|
| Device for CMI-EMR or ESD, n (%) | < 0.001 | ||
| Endoknife | 11 (32.4) | 27 (90) | |
| Tip of snare | 23 (67.6) | 3 (10) | |
| Submucosal injection solution, n (%) | < 0.001 | ||
| Saline-based solution only | 12 (35.3) | 23 (76.7) | |
| Adjuvant solutiona) | 22 (64.7) | 7 (23.3) | |
| Resection time, minutes, mean±SD | 12.7±7.0 | 18.7±11.3 | 0.011 |
| Gross en bloc resection, n (%) | 32 (94.1) | 24 (80) | 0.133 |
| Histologic complete resection, n (%) | 26 (76.5) | NAb) | NA |
| Complications | |||
| Postprocedural hemorrhage, n (%) | 1 (2.9) | 0 | 0.999 |
| Perforation, n (%) | 2 (5.9) | 0 | 0.494 |
CMI-EMR, endoscopic mucosal resection with circumferential mucosal incision; ESD, endoscopic submucosal dissection; SD, standard deviation; NA, not applicable.
a) Adjuvant solution indicates the use of sodium hyaluronate, methylcelluose, hetastarch, and/or 10% glycerol.
b) Specimens of American cases were submitted for routine histopathology in accordance with standard hospital protocols, which include neither spreading of the specimens nor 2 mm-thick slicing for histologic evaluation, and thus the histologic complete resection rate based on the microscopic evaluation of the resection margin was not available.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download