1. Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014; 384:766–781.
2. Segula D. Complications of obesity in adults: a short review of the literature. Malawi Med J. 2014; 26:20–24.
3. Grand View Research. Obesity treatment market size & forecast, by drugs (combination drugs, appetite suppressants, malabsorption & satiety drugs), by surgery & devices (adjustable gastric banding, Rouxen-Y gastric bypass, sleeve gastrectomy, biliopancreatic diversion with duodenal switch, endoscopic procedures) and trend analysis to 2024 [Internet]. San Francisco (CA): Grand View Research;c2016. [updated 2016 Sep; cited 2018 Sep 20]. Available from:
https://www.grandviewresearch.com/industry-analysis/obesity-treatment-devices-and-therapeutics-market.
4. Daniel S, Soleymani T, Garvey WT. A complications-based clinical staging of obesity to guide treatment modality and intensity. Curr Opin Endocrinol Diabetes Obes. 2013; 20:377–388.

5. Nguyen NT, Vu S, Kim E, Bodunova N, Phelan MJ. Trends in utilization of bariatric surgery, 2009-2012. Surg Endosc. 2016; 30:2723–2727.

6. Vargas EJ, Rizk M, Bazerbachi F, Abu Dayyeh BK. Medical devices for obesity treatment: endoscopic bariatric therapies. Med Clin North Am. 2018; 102:149–163.
7. Sullivan S, Edmundowicz SA, Thompson CC. Endoscopic bariatric and metabolic therapies: new and emerging technologies. Gastroenterology. 2017; 152:1791–1801.

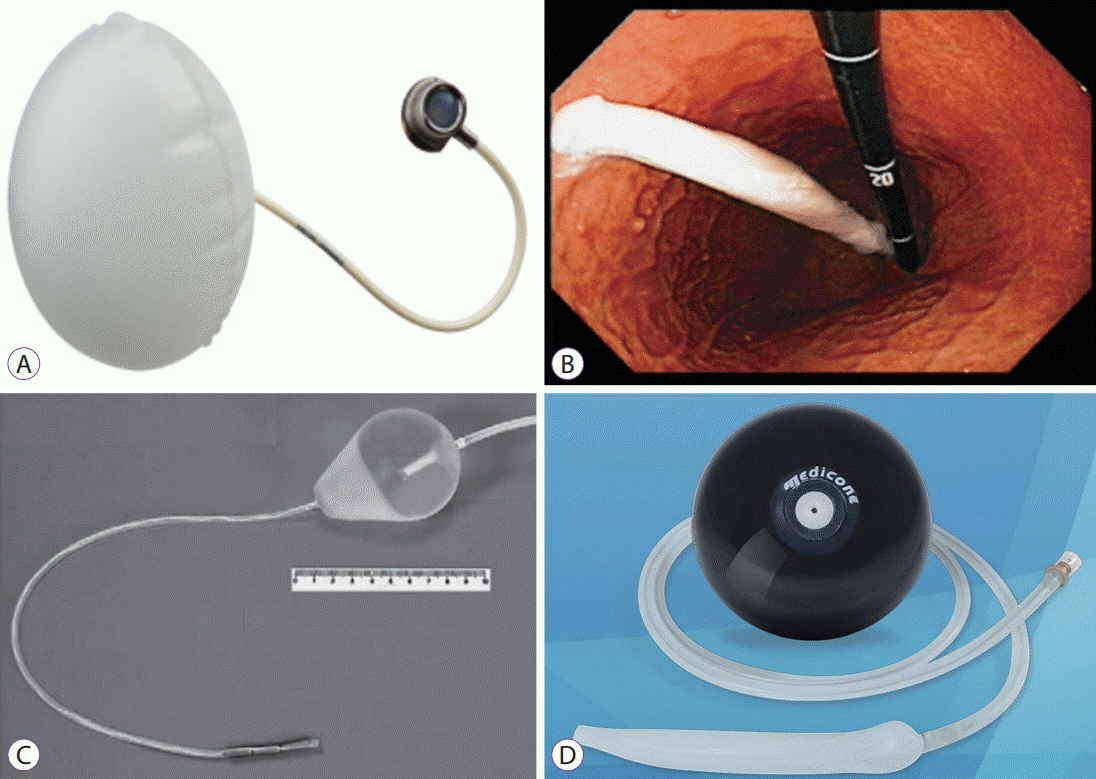
8. Kim SH, Chun HJ, Choi HS, Kim ES, Keum B, Jeen YT. Current status of intragastric balloon for obesity treatment. World J Gastroenterol. 2016; 22:5495–5504.

9. Gleysteen JJ. A history of intragastric balloons. Surg Obes Relat Dis. 2016; 12:430–435.

10. Nieben OG, Harboe H. Intragastric balloon as an artificial bezoar for treatment of obesity. Lancet. 1982; 1:198–199.
11. Gomez V, Woodman G, Abu Dayyeh BK. Delayed gastric emptying as a proposed mechanism of action during intragastric balloon therapy: results of a prospective study. Obesity (Silver Spring). 2016; 24:1849–1853.
12. Swidnicka-Siergiejko A, Wróblewski E, Andrzej D. Endoscopic treatment of obesity. Can J Gastroenterol. 2011; 25:627–633.

13. Kentish SJ, Page AJ. The role of gastrointestinal vagal afferent fibres in obesity. J Physiol. 2015; 593:775–786.

14. Brooks J, Srivastava ED, Mathus-Vliegen EM. One-year adjustable intragastric balloons: results in 73 consecutive patients in the U.K. Obes Surg. 2014; 24:813–819.

15. Machytka E, Gaur S, Chuttani R, et al. Elipse, the first procedureless gastric balloon for weight loss: a prospective, observational, open-label, multicenter study. Endoscopy. 2017; 49:154–160.

16. Lecumberri E, Krekshi W, Matía P, et al. Effectiveness and safety of airfilled balloon Heliosphere BAG® in 82 consecutive obese patients. Obes Surg. 2011; 21:1508–1512.

17. Almeghaiseeb ES, Ashraf MF, Alamro RA, Almasoud AO, Alrobayan AA. Efficacy of intragastric balloon on weight reduction: Saudi perspective. World J Clin Cases. 2017; 5:140–147.

18. Żurawiński W, Sokołowski D, Krupa-Kotara K, Czech E, Sosada K. Evaluation of the results of treatment of morbid obesity by the endoscopic intragastric balloon implantation method. Wideochir Inne Tech Maloinwazyjne. 2017; 12:37–48.

19. Keren D, Rainis T. Intragastric balloons for overweight populations-1 year post removal. Obes Surg. 2018; 28:2368–2373.
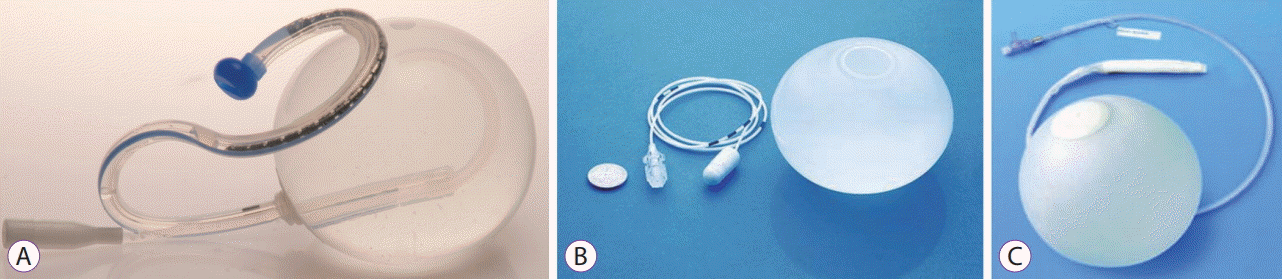
20. Gaggiotti G, Tack J, Garrido AB Jr, Palau M, Cappelluti G, Di Matteo F. Adjustable totally implantable intragastric prosthesis (ATIIP)-Endogast for treatment of morbid obesity: one-year follow-up of a multicenter prospective clinical survey. Obes Surg. 2007; 17:949–956.
21. Carvalho GL, Barros CB, Okazaki M, et al. An improved intragastric balloon procedure using a new balloon: preliminary analysis of safety and efficiency. Obes Surg. 2009; 19:237–242.

22. Lopasso FP, Sakai P, Gazi BM, et al. A pilot study to evaluate the safety, tolerance, and efficacy of a novel stationary antral balloon (SAB) for obesity. J Clin Gastroenterol. 2008; 42:48–53.

23. Martin CK, Bellanger DE, Rau KK, Coulon S, Greenway FL. Safety of the Ullorex oral intragastric balloon for the treatment of obesity. J Diabetes Sci Technol. 2007; 1:574–581.

24. Ponce J, Woodman G, Swain J, et al. The REDUCE pivotal trial: a prospective, randomized controlled pivotal trial of a dual intragastric balloon for the treatment of obesity. Surg Obes Relat Dis. 2015; 11:874–881.

27. Borges AC, Almeida PC, Furlani SMT, Cury MS, Gaur S. Intragastric balloons in high-risk obese patients in a Brazilian center: initial experience. Rev Col Bras Cir. 2018; 45:e1448.

28. Machytka E, Klvana P, Kornbluth A, et al. Adjustable intragastric balloons: a 12-month pilot trial in endoscopic weight loss management. Obes Surg. 2011; 21:1499–1507.

29. Russo T, Aprea G, Formisano C, et al. BioEnterics intragastric balloon (BIB) versus spatz adjustable balloon system (ABS): our experience in the elderly. Int J Surg. 2017; 38:138–140.

30. Daniel F, Abou Fadel C, Houmani Z, Salti N. Spatz 3 adjustable intragastric balloon: long-term safety concerns. Obes Surg. 2016; 26:159–160.

31. Bazerbachi F, Vargas Valls EJ, Abu Dayyeh BK. Recent clinical results of endoscopic bariatric therapies as an obesity intervention. Clin Endosc. 2017; 50:42–50.

32. Alsabah S, Al Haddad E, Ekrouf S, Almulla A, Al-Subaie S, Al Kendari M. The safety and efficacy of the procedureless intragastric balloon. Surg Obes Relat Dis. 2018; 14:311–317.

33. Angrisani L, Santonicola A, Vitiello A, Belfiore MP, Belfiore G, Iovino P. Elipse balloon: the pitfalls of excessive simplicity. Obes Surg. 2018; 28:1419–1421.

34. Trande P, Mussetto A, Mirante VG, et al. Efficacy, tolerance and safety of new intragastric air-filled balloon (Heliosphere BAG) for obesity: the experience of 17 cases. Obes Surg. 2010; 20:1227–1230.

35. Caglar E, Dobrucali A, Bal K. Gastric balloon to treat obesity: filled with air or fluid? Dig Endosc. 2013; 25:502–507.

36. De Castro ML, Morales MJ, Del Campo V, et al. Efficacy, safety, and tolerance of two types of intragastric balloons placed in obese subjects: a double-blind comparative study. Obes Surg. 2010; 20:1642–1646.

37. Giardiello C, Borrelli A, Silvestri E, Antognozzi V, Iodice G, Lorenzo M. Air-filled vs water-filled intragastric balloon: a prospective randomized study. Obes Surg. 2012; 22:1916–1919.

38. Drozdowski R, Wyleżoł M, Frączek M, Hevelke P, Giaro M, Sobanski P. Small bowel necrosis as a consequence of spontaneous deflation and migration of an air-filled intragastric balloon - a potentially life-threatening complication. Wideochir Inne Tech Maloinwazyjne. 2014; 9:292–296.

39. Bužga M, Evžen M, Pavel K, et al. Effects of the intragastric balloon MedSil on weight loss, fat tissue, lipid metabolism, and hormones involved in energy balance. Obes Surg. 2014; 24:909–915.

40. Buzga M, Kupka T, Siroky M, et al. Short-term outcomes of the new intragastric balloon End-Ball® for treatment of obesity. Wideochir Inne Tech Maloinwazyjne. 2016; 11:229–235.

41. Pezzo CT, de Souza TF, Fenero V, Suano-Souza FI, Grecco E, Sarni ROS. Efficacy and safety of intragastric balloon in the treatment of obesity in adolescent females. Nutrire. 2017; 42:26.

42. Neto MG, Silva LB, Grecco E, et al. Brazilian intragastric balloon consensus statement (BIBC): practical guidelines based on experience of over 40,000 cases. Surg Obes Relat Dis. 2018; 14:151–159.

43. Do TN, Ho KY, Phee SJ. A magnetic soft endoscopic capsule-inflated intragastric balloon for weight management. Sci Rep. 2016; 6:39486.

44. Ghoz HM, Acosta A, Topazian M, Camilleri M, Gostout C, Dayyeh BKA. A pre-clinical animal study of combined intragastric balloon and duodenal-jejunal bypass sleeve for obesity and metabolic disease. Gastroenterology. 2016; 150(4 Suppl 1):S231–S232.
45. Choi HS, Chun HJ. Recent trends in endoscopic bariatric therapies. Clin Endosc. 2017; 50:11–16.





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download