INTRODUCTION
INTRODUCTION TO LCI AND BLI
Wavelength and color information in endoscopic systems
Table 1.
Color recognition of upper gastrointestinal lesions for screening
Selecting LCI or BLI for screening
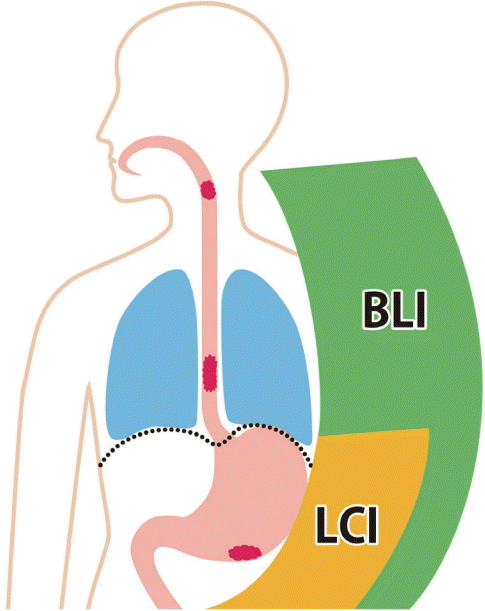 | Fig. 1.Different applications of linked color imaging (LCI) and blue laser imaging (BLI, or BLI-bright) in upper gastrointestinal cancer screening. BLI is better for visualizing the regions from the pharynx to lower esophagus. LCI is better for the visualizing the area from the esophagogastric junction (e.g., Barrett’s esophagus) to the duodenum where BLI (or BLI-bright) is also useful at near views. |
The importance of color to establish a diagnosis
ESOPHAGUS
Superiority of BLI in detecting squamous cell carcinoma
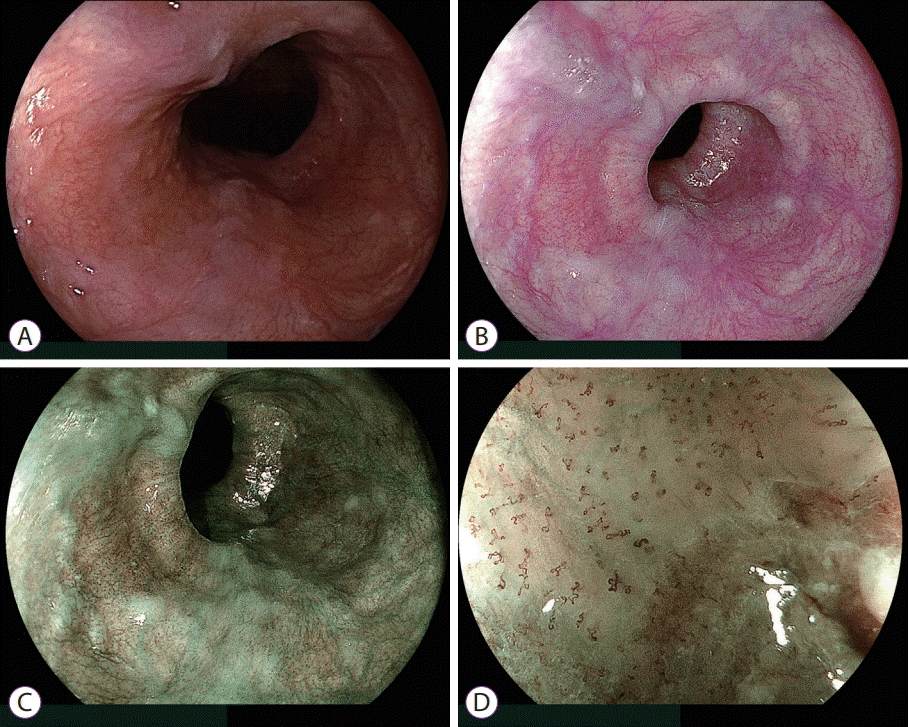 | Fig. 2.Esophageal squamous cell carcinoma. (A) White light imaging and (B) linked color imaging do not demonstrate abnormal features suggestive of a malignancy. (C) Blue laser imaging highlights a brown malignant lesion, distinct from the surrounding mucosa. (D) Magnifying blue laser imaging shows irregular intrapapillary capillary loops suggestive of cancer. |
Findings in Barrett’s esophagus and adenocarcinoma
STOMACH
Early gastric cancer with chronic H. pylori infection
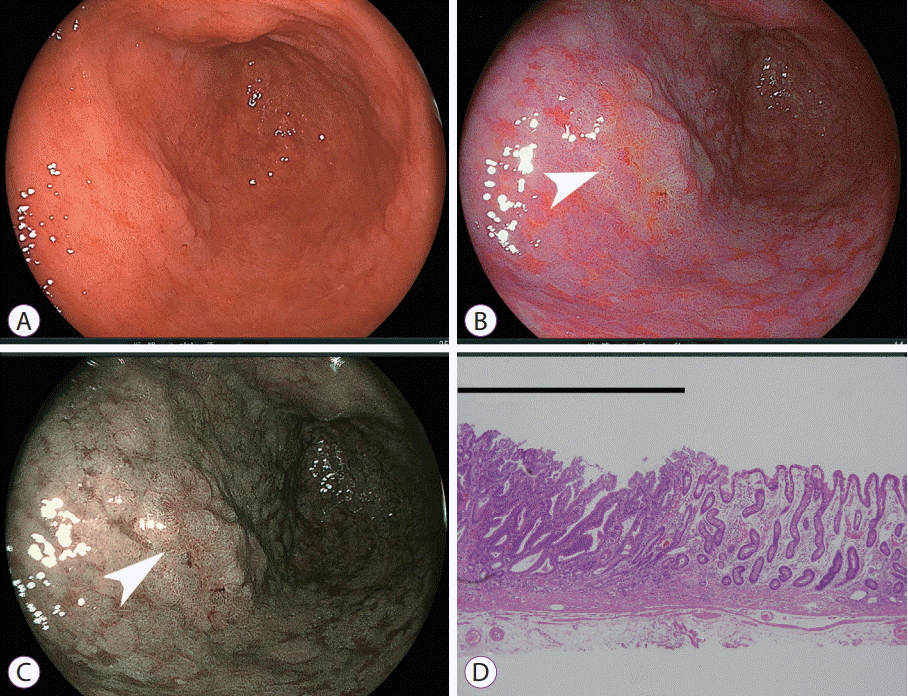 | Fig. 3.Early gastric cancer in the antrum where intestinal metaplasia spreads over broad areas. (A) White light imaging does not demonstrate findings suggestive of malignancy. (B) Linked color imaging shows an orange lesion (arrow), suggestive of cancer, surrounded by purple mucosa. (C) Blue laser imaging-bright shows a brown lesion (arrow) surrounded by green mucosa. (D) Pathological evaluation of the resected specimen shows well-differentiated adenocarcinoma (black line) adjacent to intestinal metaplasia (hematoxylin and eosin, ×40). |
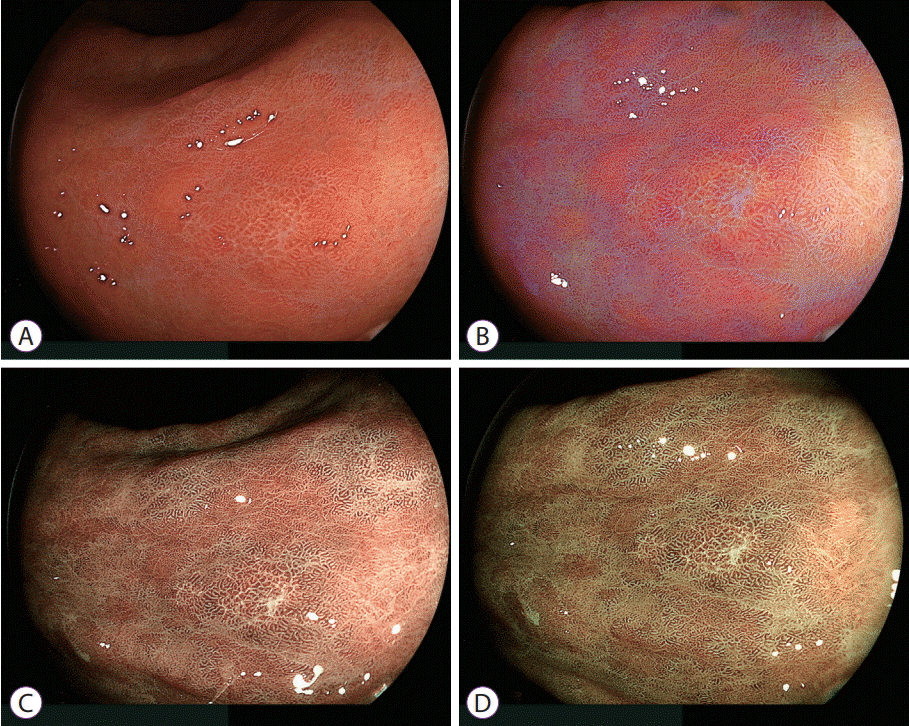 | Fig. 4.Comparison between C1 and C2 green enhancement of mucosa surrounding an early gastric cancer. (A) White light imaging shows a slightly discolored lesion. (B) Linked color imaging shows an orange lesion partially surrounded by purple mucosa. In addition, purple areas have a patchy distribution in the background mucosa. (C) Blue laser imaging with C1 color enhancement shows that the purple mucosae demonstrated by linked color imaging have changed to pale green. (D) Blue laser imaging with C2 color enhancement changes the mucosal color to dense green which highlights the apparent line of demarcation between the brown malignant lesion and surrounding mucosa. |
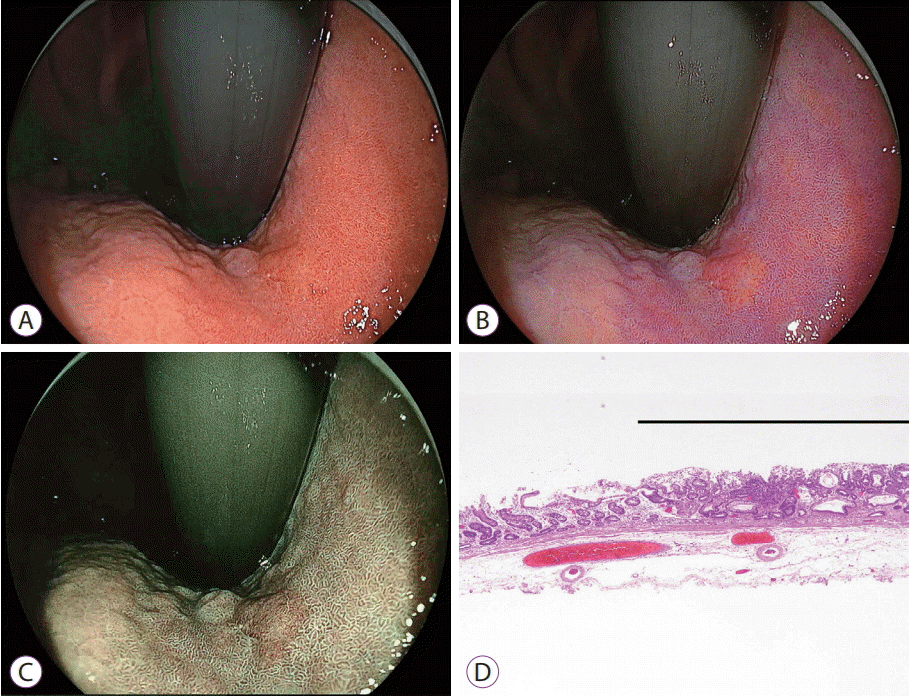 | Fig. 5.Early gastric cancer near the cardia. (A) White light imaging shows slightly irregular surface mucosa that has the same color as the surrounding mucosa. (B) Linked color imaging shows an orange lesion surrounded by purple mucosa. (C) Blue laser imaging shows a brown lesion surrounded by green mucosa. (D) Pathological evaluation of the resected specimen shows well-differentiated adenocarcinoma (black line) adjacent to intestinal metaplasia (hematoxylin and eosin, ×40). |
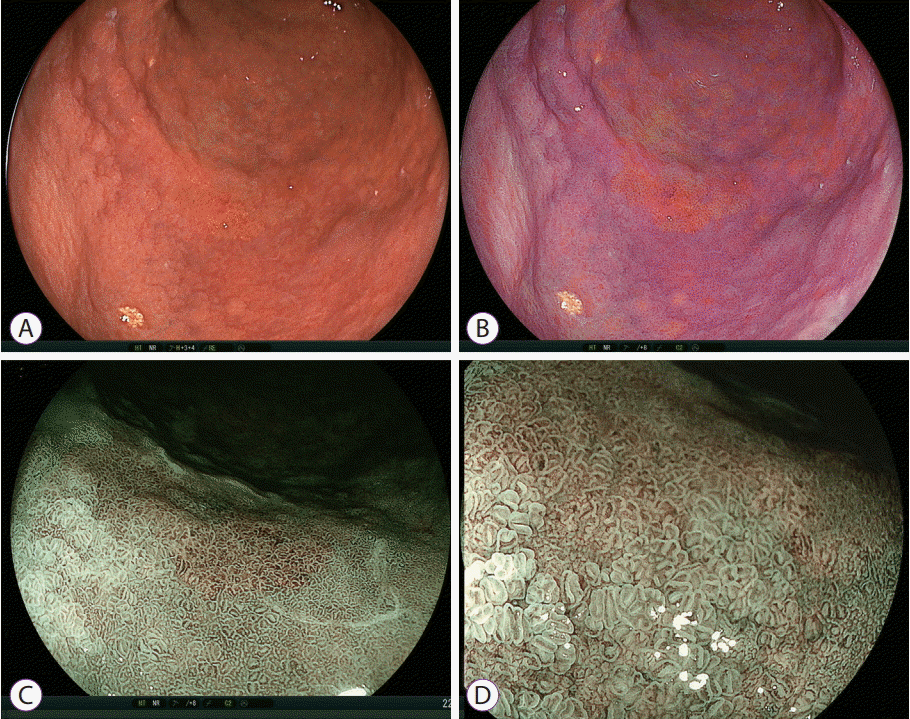 | Fig. 6.Early gastric cancer after Helicobacter pylori eradication. (A) White light imaging shows a slightly irregular lesion on the greater curvature. (B) Linked color imaging shows an orange lesion surrounded by purple mucosa. In addition, purple areas have patchy and flat-sheet distributions in the background mucosa. Blue laser imaging (C) at close and (D) magnified views shows a brown lesion surrounded by green mucosa. However, the surface patterns are similar in the brown malignant lesion and the surrounding mucosa because this malignant lesion occurred after H. pylori eradication. |
Detecting early gastric cancers in areas of intestinal metaplasia
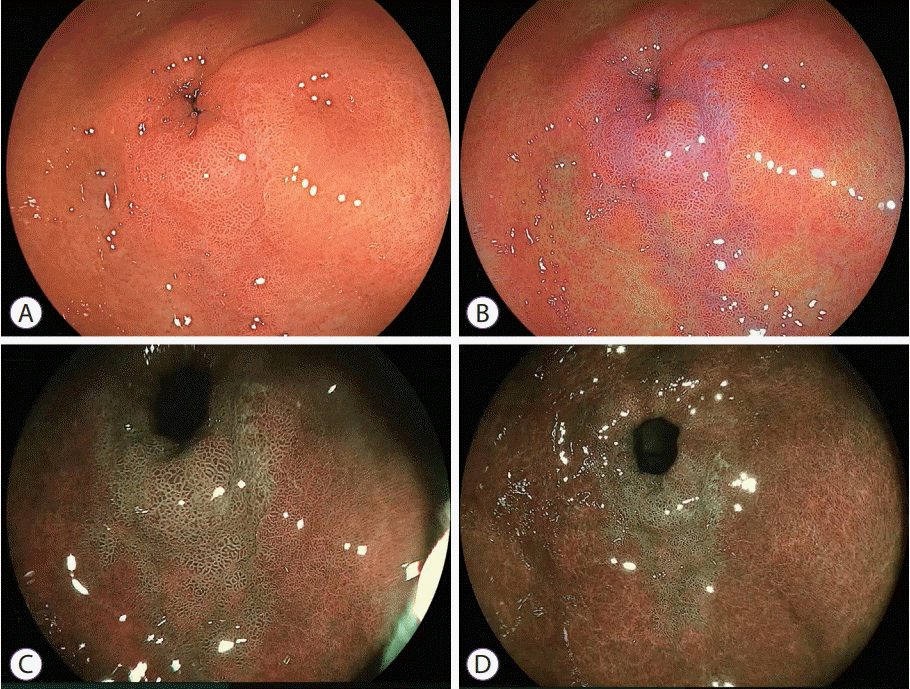 | Fig. 7.Intestinal metaplasia near the pyloric ring. (A) White light imaging does not demonstrate abnormal features. (B) Linked color imaging shows purple mucosa near the pyloric ring. (C) Blue laser imaging-bright and (D) blue laser imaging visualize a green lesion, suggesting intestinal metaplasia. |
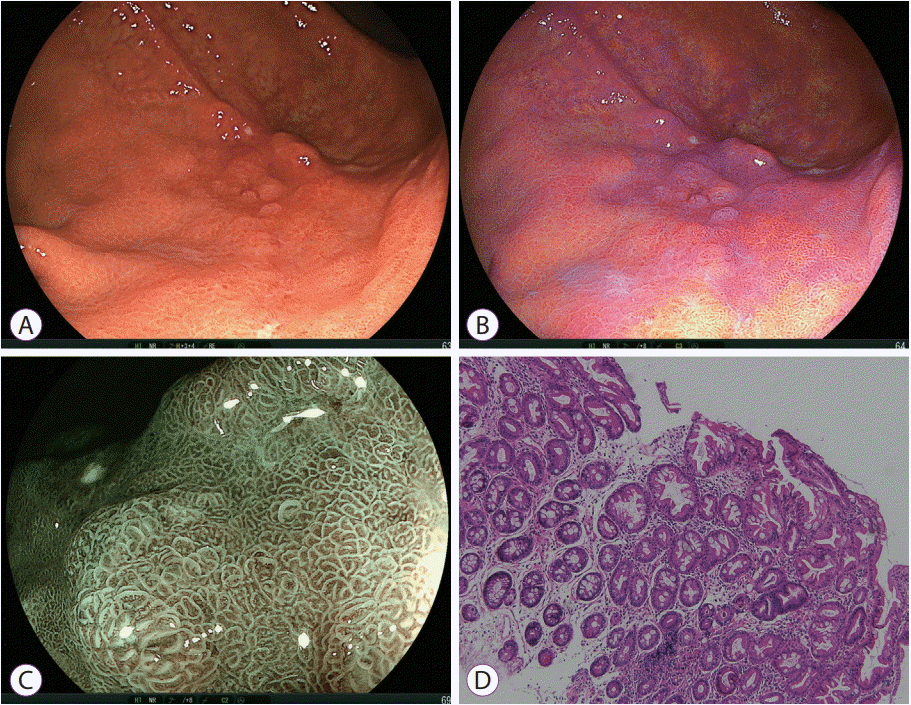 | Fig. 8.Gastric intestinal metaplasia. (A) White light imaging shows an irregular depressed lesion, suggesting advanced gastric cancer or mucosa-associated lymphoid tissue lymphoma on the greater curvature. However, (B) linked color imaging shows a purple lesion homogenously spreading in the depressed area, suggesting intestinal metaplasia. (C) Blue laser imaging shows a regular surface pattern with green circle lines corresponding to the marginal crypt epithelium, suggesting intestinal metaplasia. (D) Pathological evaluation of the biopsy specimen shows intestinal metaplasia (hematoxylin and eosin, ×40). |
Early gastric cancer after H. pylori eradication
Detecting early gastric cancers among erosions or multiple tiny gastric lesions
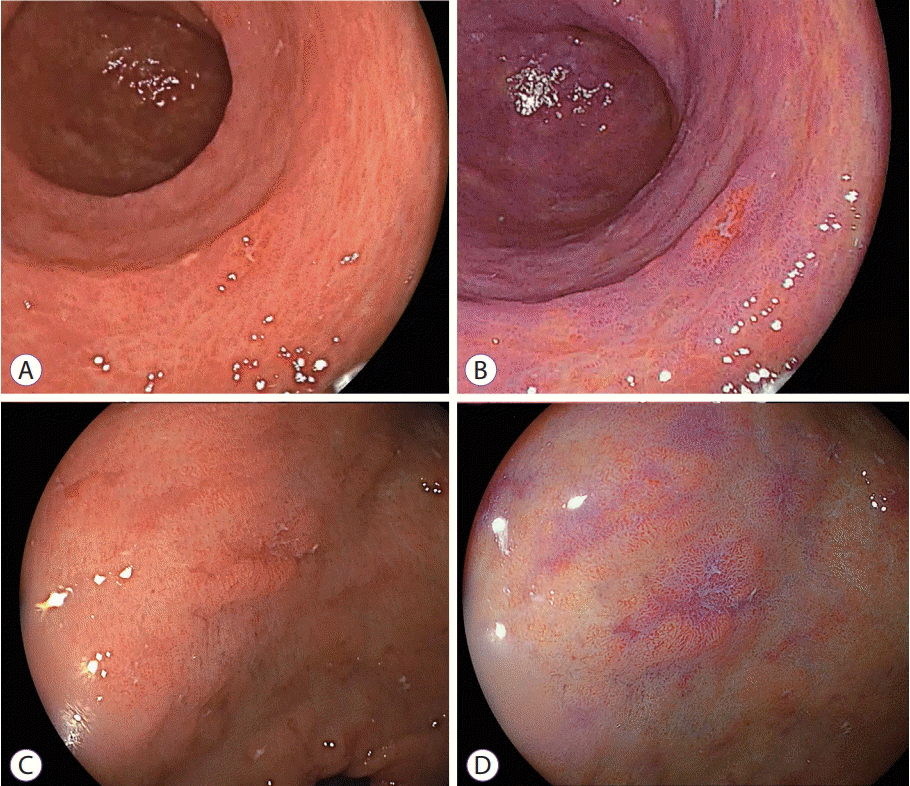 | Fig. 9.Differentiating gastric cancer and an erosion by linked color imaging. The erosion is observed in the antrum by (A) white light imaging and changes to (B) orange-reddish mucosa using linked color imaging, suggesting cancer. Similarly, an erosion is observed in the antrum by (C) white light imaging but changes to (D) purple mucosa using linked color imaging, suggesting inflamed mucosa. |
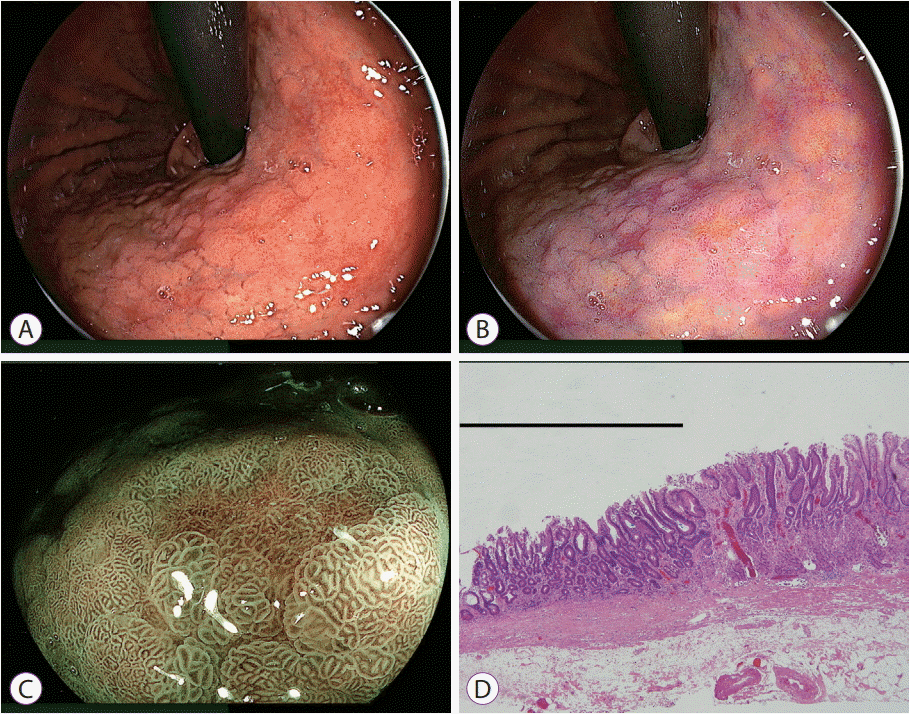 | Fig. 10.Detection of gastric cancer among multiple depressed lesions by linked color imaging. (A) White light imaging shows multiple depressed lesions in the gastric body. (B) Linked color imaging shows many depressed purple lesions suspicious for inflamed mucosa such as intestinal metaplasia and only one orange lesion suspicious for cancer (central portion of the image). (C) The orange lesion changes to brown with an irregular surface pattern surrounded by green mucosa using blue laser imaging, suggesting cancer. (D) Pathological evaluation of the resected specimen shows well-differentiated adenocarcinoma (black line) adjacent to intestinal metaplasia (hematoxylin and eosin, ×40). |
Early gastric cancer without H. pylori infection
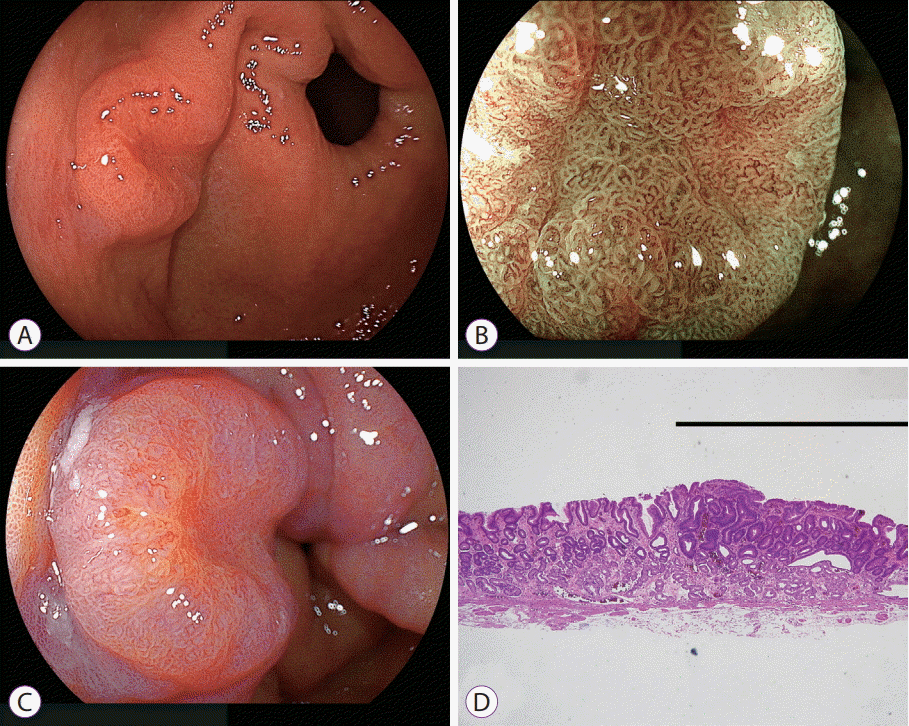 | Fig. 11.Early gastric cancer in normal background mucosa not infected with Helicobacter pylori using linked color imaging. (A) White light imaging shows an elevated lesion near the pyloric ring. (B) Blue laser imaging at low magnification shows a relatively regular surface pattern in the central portion. However, (C) linked color imaging shows an orange lesion on the mucosal surface, suggestive of cancer. (D) Pathological evaluation of the resected specimen shows well-differentiated adenocarcinoma (black line) (hematoxylin and eosin, ×40). |
Undifferentiated gastric cancer
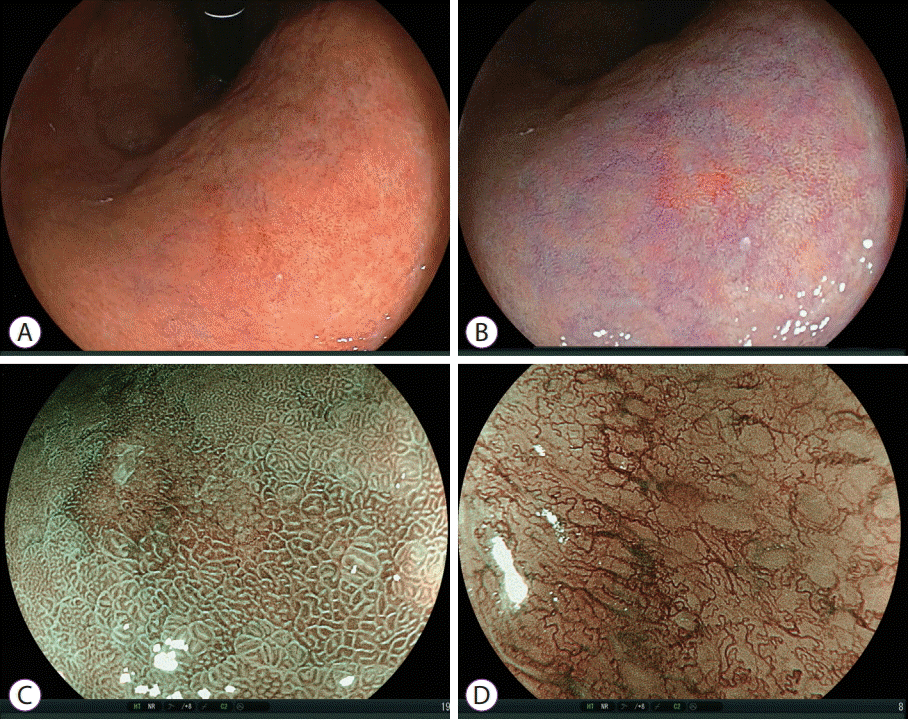 | Fig. 12.Diagnosis of undifferentiated gastric carcinoma by linked color imaging and blue laser imaging. (A) It is difficult to identify malignant lesions using white light imaging. (B) Linked color imaging demonstrates a small orange-reddish lesion with a normal surface pattern. (C) Blue laser imaging shows a brown lesion with an irregular surface pattern surrounded by green mucosa. (D) Magnifying blue laser imaging shows irregular microvessels in an unstructured area suggesting undifferentiated carcinoma. |
Transnasal endoscopy with LCI in detecting early gastric cancer
DUODENUM
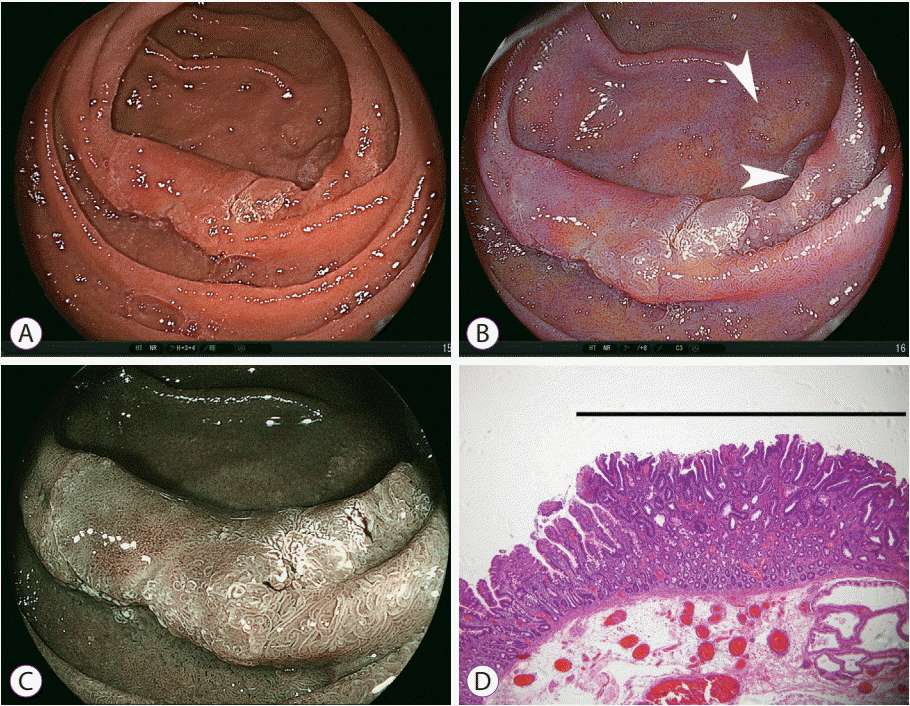 | Fig. 13.Imaging of early duodenal cancer and multiple adenomas in the descending portion of the duodenum using linked color imaging. (A) White light imaging shows an elevated lesion in the descending portion, which accompanies a central red area and peripheral white area. (B) Linked color imaging shows an orange main lesion in the central area, suggesting cancer, and two enhanced white lesions in the satellite areas, suggesting adenoma (white arrows). (C) Blue laser imaging of the main lesion shows a brown lesion in the central area, suggesting cancer. (D) Pathological evaluation of the resected specimen shows well-differentiated adenocarcinoma (black line) in the elevated portion of the main lesion (hematoxylin and eosin, ×40). |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download