Abstract
Colon capsule endoscopy (CCE) is a relatively new diagnostic procedure for patients with suspected colonic diseases. This convenient, noninvasive method enables the physician to explore the entire colon without significant discomfort to the patient. However, while CCE can be performed painlessly without bowel air insufflation, the need for vigorous bowel preparation and other technical limitations exist. Due to such limitations, CCE has not replaced conventional colonoscopy. In this review, we discuss historical and recent advances in CCE including technical issues, ideal bowel preparation, indications and contraindications and highlight further technical advancements and clinical studies which are needed to develop CCE as a potential diagnostic tool.
Colonoscopy is the gold standard for diagnosing lower gastrointestinal diseases. Colonoscopy facilitates simultaneous visualization of the colon mucosa, biopsy, and resection of polyps or early cancer. While the clinical value of colonoscopy is well established, many patients regard colonoscopy as an unpleasant experience. Further, severe complications such as bowel perforation or bleeding, although rare, can develop during colonoscopy [1]. In a Canadian study, about 52% of female patients reported anxiety, with 29% reporting high level anxiety due to colonoscopy [2]. Anxiety and unpleasant memories from previous colonoscopy can influence the patient’s compliance with colonoscopy surveillance in the future.
Video capsule endoscopy (VCE) was first developed as a tool for small bowel evaluation in 2000 [3]. The endoscopic images of the mid small bowel was relatively unseen until the US Food and Drug Administration approved the use of VCE in 2001. VCE is now widely used to evaluate the small bowel mucosa under direct inspection [4]. Increasing evidence supporting the use of VCE for small bowel evaluation has led to great interest in colon capsule endoscopy (CCE). The first generation CCE (PillCam-Colon; Given Imaging Ltd., Yoqneam, Israel) was open to the public in 2006 [5]. Since then, numerous studies have reported the clinical efficacy of CCE. We will comprehensively review previous studies and discuss the current status and future direction of CCE.
Given Imaging Ltd. developed a new capsule to evaluate the entire colon in 2006 [5]. The first generation CCE (CCE-1) was similar in appearance to conventional VCE, although it had two cameras, one at both ends. The view angle from both cameras is 156°, and each camera can be used to obtain 2 images per second. The size of CCE-1 was 11×31 mm, which is slightly longer than small bowel VCE (Fig. 1). In order to save battery before exploring the colon, CCE-1 was maintained in a ‘sleep mode’ after recognizing the esophagus and stomach for 1 h and 45 min. During this time, capsule passes most of the small bowel and reaches the terminal ileum. After this sleeping time, CCE-1 automatically reactivates and evaluates the entire colon. Similar to small bowel VCE, a dedicated external portable recorder (DR2C) receives and stores images from CCE-1. After completion of the examination, DR2C is linked to workstation and reconstruction of images is processed by proprietary (“RAPID”) software.
Second generation CCE (CCE-2) manufactured by Given Imaging Ltd. was presented in 2009 [6]. In addition to a slightly larger size (11.6×31.5 mm) compared with CCE-1, CCE-2 carries several additional features (Fig. 1). First, CCE-2 cameras contain wider angles (up to 172°), enabling nearly 360° imaging of the colonic mucosa. Second, CCE-2 has an adaptive image acquisition rate depending on the speed of capsule propulsion. CCE-2 captures 35 frames per second during active movement of capsule, while 4 frames per second are captured during the stationary period of capsule movement. Third, CCE-2 has a different battery saving system. Until small bowel images are detected, CCE-2 captures only 14 images per minute. Fourth, CCE-2 facilitates high resolution imaging below 0.1 mm, with a magnification of about 1 to 8. The use of additional software, such as the Flexible Spectral Imaging Color Enhancement, further improves the detection rate of colon lesions.
Lastly, the smart, new data recorder (DR3) contains an alarm system, which assists patients and the medical team. DR3 recognizes the position of the capsule and sends visual/audio signals at the time of taking booster medications for bowel preparation. Booster cleansing is very important because CCE transits the long small bowel and images the colon during its battery time [7]. Since CCE lacks an air inflation and suction system, any residual stool may interrupt precise reading of captured images of colon.
These technical advances have enabled CCE to navigate through the entire colon and efficiently detect lesions. Although several practical limitations and technical issues persist, there is clear and ongoing improvement of CCE.
Considering the absence of air inflation and suction, clinical success of CCE highly depends on the degree of bowel cleansing. Accurate detection of polyps or inflammation can only be achieved in a completely clean colon without any kind of debris. The European Society of Gastrointestinal Endoscopy (ESGE) and American Society of Gastrointestinal Endoscopy guidelines recommend a split-dose regimen of at least 4 liters of polyethylene glycol (PEG) in the evening before and during the morning of the colon study. However, a 4-liter PEG regimen cannot guarantee a perfectly clean bowel [8]. Spada et al. demonstrated the effectiveness of sodium-phosphate (NaP) booster [9]. When compared to the PEG regimen, NaP booster resulted in excellent bowel cleansing and adequate bowel distention with clear liquid and propulsion of the capsule. The propulsion of the capsule within the battery time is an important factor for the success of CCE [9].
Another European study utilized a regimen composed of 4 liters of PEG + prokinetics (domperidone) + two-part NaP booster (45 mL and 30 mL) + bisacodyl [10]. In this study, in which the primary end-point was the polyp detection rate of CCE compared with conventional colonoscopy, adequate bowel cleansing was achieved in 72% of patients in the CCE group, compared with 87% in the colonoscopy group (p<0.001) [10]. Using the same regimen, Schoofs et al. reported a 77% adequate preparation [11]. A meta-analysis of this regimen reported an excellent-to-good level of preparation in 77% (median value) [12]. Of note, all of these are below the current standards for quality in colonoscopy of at least 90% adequate (good or excellent) preparation, likely due to the inability was wash and suction as is commonly done at colonoscopy [13]. As mentioned above, capsule excretion within battery time is another vital factor during CCE. With the use of this regimen, Van Gossum et al. and Gay et al. reported a 90.5% to 92.8% excretion rate within the battery time [10,14]. In terms of quality control, a 90% excretion rate of CCE is comparable to 95% rate of cecal intubation during colonoscopy. One of the most extensively used preparation regimens for CCE is described in Table 1 [10,11].
Due to major complications associated with NaP including electrolyte disturbance, acute nephropathy and kidney failure, several studies examined the efficiency of other modified booster regimens [9,15]. Unfortunately, most studies failed to report positive results. Recently, a Spanish group compared a one-day versus two-day cleansing regimen using a reduced volume of NaP booster [16]. The one-day group received a fiber-free diet and 3 liters of PEG on day 0, while the two-day group received a liquid diet and 3 liters of PEG in the evening of day -1, and 1 liter of PEG in the early morning of day 0. In both groups, bisacodyl 15 mg was used on day -1. Further, NaP booster was added once or twice after capsule ingestion. The authors reported 94% of adequate bowel cleansing in the one-day schedule group, and 84% in the two-day schedule group. However, there was no statistical difference between groups (p=0.27).
Another study using PEG plus ascorbic acid without NaP booster showed adequate level of bowel cleansing [17]. The patients in the original group received 1 liter of Moviprep (Norgine GmBH, Marburg, Germany) in the evening prior to CCE, followed by 0.75 liter of Moviprep in the morning of CCE. Booster regimen was also provided with Moviprep (0.5 L and 0.25 L). Domperidone and bisacodyl suppositories were used. Patients in the control group received 1 liter Moviprep on the morning of CCE and other regimen remained the same as in the original group. The study reported that 83% of original group and 82% of control group showed good to excellent bowel cleansing, suggesting the utility of PEG plus ascorbic acid as a preparation regimen in CCE.
Spada et al. proposed a regimen with reduced NaP volume [18]. Unlike prior studies, Spada et al. used 30 mL and 15 mL NaP booster with water [18]. The overall adequate bowel preparation was 78% and the colon excretion rate was 83%. While the results were comparable to other studies, adequate cleansing of CCE at the cecum was just 50%. Adequate cleansing of colonoscopy at cecum was 70% in the same study. Adequate bowel cleansing of the right colon was also quite low in the CCE group (65%). The US multi-society task force on colorectal cancer (CRC) set a target cecal intubation of at least 90%, but failure rate of cecal intubation was about 13% in real practice [19]. Since failed colonoscopy leads to failed observation of the proximal colon, appropriate bowel cleansing and expansion are very important. Colonoscopy failure is an indication for CCE.
In the several studies regarding bowel preparation of CCE that were reviewed, bowel preparation requires as much as 6 liters of fluid over two days. In real clinical practice, the large volume of PEG and water is a challenge to the patients. Kakugawa et al. suggested reducing the volume of fluid preparation [20]. Patients in the group treated with reduced volume method abstained from drink PEG the day before procedure, while they consumed 2 liters of PEG during the day of procedure. The booster regimen comprised magnesium citrate 100 mg plus 900 mL water. Compared with the conventional volume group, reduced volume group reported comparable and adequate bowel preparation (94% of reduced group vs. 86% conventional group). Although not significant, the capsule excretion rate within the battery time was quiet low (71% in the reduced group and 55% in the conventional group).
CCE has inherent technical limitations. The current technology does not allow tissue extraction, inflation of air, suction of debris, or movement control. Thus, adequate bowel cleansing and expansion to ensure a “submarine view” are the most important factors for CCE success. Development of clean and expanded bowel with a reduced volume of preparation fluids is needed for successful CCE.
Essentially, CCE was designed to evaluate colonic mucosa, making the indications of CCE not much different from that of colonoscopy. All suspected or known colonic diseases are indications for CCE [21]. Most studies regarding CCE compared conventional diagnostic tools such as colonoscopy or computed tomographic colonoscopy with CCE in colonic diseases [5-14,16-18]. Screening of colorectal neoplasms, monitoring and diagnosis of inflammatory bowel diseases (IBDs), and incomplete colonoscopy or unwilling to perform colonoscopy are indications for CCE (Fig. 2). Detailed reports about each indication will be described in a later chapter. Contraindications for CCE are similar to that of VCE [22]. They include swallowing disorders, prior abdominal surgery of gastrointestinal tract, known or suspected bowel obstruction, presence of cardiac pacemaker and pregnancy. History of abdominal surgery and bowel obstruction may be related to capsule retention. Signals from CCE may interrupt cardiac pacemaker action and microwave from the CCE may be a threat to pregnancy [23]. Risk of aspiration may be increased in patients with swallowing disorder. Physicians should select candidates for CCE depending on the patient’s condition, based on the indications and contraindications of CCE.
Colonoscopy is the gold standard for screening of CRCs and the diagnosis of colorectal pathologies. However, incomplete colonoscopies have been reported due to intestinal obstruction, acute angulations, adhesions due to past abdomino-pelvic surgery, hernias, or patient intolerance. Patients with incomplete colonoscopy are usually referred to computed tomographic colonography (CTC), especially in case of bowel obstruction due to CRC. CTC may provide information about colorectal lesions as well as extra-colonic findings [24]. Previous studies reported a CTC sensitivity of 82% to 92%. for colorectal tumors ≥1 cm in size [24]. Although the complications of CTC are very rare, CTC is associated with potential risk of colonic perforation and radiation [25]. Furthermore, the sensitivity of CTC is less than that of colonoscopy for the detection of polyps <1 cm in size [26,27], flat [28], and serrated lesions [29]. Two studies comparing CCE and CTC suggested that CCE was as good as [30], or better than CTC [31] for CRC screening. In patients with incomplete colonoscopy, the relative sensitivity of CCE compared with CTC was 2.0 (95% confidence interval [CI], 1.34 to 2.98), indicating a significant increase in sensitivity for lesions ≥6 mm [31]. In addition, a survey for adherence to CRC screening suggests that CCE may have a positive impact on adherence rates [32]. In patients who are unwilling, unable, or inappropriate for CTC. CCE is an acceptable alternative in cases without intestinal obstruction.
Meta-analyses [12,33] and subsequent studies [14,34] indicated that CCE was accurate for the detection of colorectal neoplasia (CRN), less sensitive and specific than conventional colonoscopy. In the meta-analyses, the per-patient sensitivities and specificities of CCE compared with conventional colonoscopy for detection of any polyp were 71%–73% and 75%–89%, and 68%–69% and 82%–86% for the detection of significant polyps (≥6 mm and/or ≥3 polyps), respectively [12,33]. However, studies of CCE-1 showed a significant heterogeneity in study design, patient population, and CCE performance characteristics [5,10-14,33,35-37]. In studies with CCE-2, the reported sensitivities and specificities for detection of any polyp were 82% and 86%, and for the detection of significant polyps (≥6 mm) were 84%–89% and 64%–88%, respectively [6,30,38-44]. The sensitivity and specificity of CCE for detection of polyps are listed in Table 2. The US Food and Drug Administration approved CCE for patients after incomplete optical colonoscopy as well as for patients with major risks for colonoscopy or moderate sedation (Jonette R. Foy, PhD, e-mail communication, Feb 2014). Japan’s Pharmaceuticals and Medical Devices Agency has approved a CCE for the diagnosis of colonic disease in patients unwilling or unable to undergo colonoscopy [45]. The ESGE recommended CCE as a feasible and safe tool for visualization of the colon in patients with incomplete colonoscopy without obstruction [7].
Theoretically, CCE is a noninvasive modality for the assessment of the extent and severity of IBD. However, the role of CCE as a primary diagnostic modality is limited, because biopsy and histological diagnosis are mandatory for the initial diagnosis of IBD [23]. Similarly, CCE may not be inappropriate for the surveillance of CRC in asymptomatic patients with IBD. Currently, conventional colonoscopy recommends initial IBD diagnosis and surveillance of colitis-associated cancers, because of high sensitivity for the detection of mucosal lesions and ability to obtain biopsy from the suspected lesions.
The outcome of CCE compared with ileo-colonoscopy in patients with IBD is listed in Table 3. In an early prospective study using CCE-1, the sensitivity and specificity of CCE-1 for the detection of active colonic inflammation in patients with known or suspected ulcerative colitis (UC) were 89% and 75%, respectively [46]. Other studies using CCE-1 reported acceptable safety but insufficient efficacy as an alternative to conventional colonoscopy for monitoring disease extent and activity [47,48]. Nonetheless, in a recent prospective study, CCE-2 reported a sensitivity of 97% and 94% to detect mucosal inflammation (Mayo endoscopic subscore >0) and moderate-to-severe inflammation (Mayo endoscopic subscore >1) [49], respectively. The negative predictive values of CCE-2 to detect mucosal inflammation reached 94% to 95%, respectively [49]. In pediatric UC patients, CCE-2 showed acceptable efficacy with sensitivity and specificity for disease activity of 96% and 100%, respectively [50]. ESGE guidelines recommended that CCE-2 may facilitate the monitoring of mucosal inflammation in patients with UC [7].
Data regarding the use of CCE are insufficient in patients with Crohn’s disease (CD). In patients with active CD of the colon, CCE-2 underestimated the severity of disease compared with colonoscopy, and detected colonic ulcerations with 86% sensitivity and 40% specificity, respectively [51]. The diagnostic accuracy of CCE for active CD lesion was comparable to that of colonoscopy [52,53]. When pan-enteric capsule endoscope was used, the diagnostic yield of small-bowel colon capsule was superior to ileo-colonoscopy (83.3% vs. 69.7%; yield difference, 13.6%; 95% CI, 2.6%–24.7%) Among the 66 enrolled patients, 43 were diagnosed with active CD disease by both modalities, and 12 subjects tested positive for active CD using small-bowel colon capsule alone (5 subjects showed active CD lesions in the terminal ileum), and 3 subjects tested positive for active CD with ileo-colonoscopy [54]. CCE appears to underestimate the extent and severity of disease compared with colonoscopy in patients with CD. The American Gastroenterological Association guidelines recommend against substituting CCE for colonoscopy to assess the extent and severity of disease in patients with IBD [55]. Recently, mucosal healing considered as a treatment target of IBD for improved clinical outcome and endoscopic monitoring have become important parameters to assess disease activity. CCE was associated with better tolerance and was preferred by patients compared with conventional colonoscopy. Based on the recent evidence, CCE-2 may be useful for the assessing the severity of UC [56]. The monitoring of inflammation may facilitate therapeutic decision-making, and further studies are needed to support this strategy.
In the meta-analysis, the adverse events from CCE occurred in 4.1% (95% CI, 2.6%–5.6%) of cases, although these were mild-to-moderate, such as nausea and abdominal pain [12]. The risks of CCE include complications associated with bowel preparation, aspiration, and capsule retention. The risks associated with bowel preparation include nausea with and without vomiting, abdominal pain, rare pulmonary aspiration, Mallory-Weiss tear, pancreatitis, colitis, lavage-induced pill malabsorption, and cardiac arrhythmia [57].
Capsule retention is the most serious complication of CCE similar to small bowel capsule endoscopy. Capsule retention refers to capsule remaining in the gastrointestinal tract for at least two weeks. The incidence of capsule retention is about 2% of all patients undergoing small bowel capsule endoscopy. Capsule retention should be suspected in all asymptomatic patients who do not report capsule excretion within 15 days of capsule ingestion; and in patients with obstructive or perforation-related symptoms in which the capsule has not been excreted, regardless of the time between the onset of symptoms and capsule ingestion [57]. In IBD patients, especially established CD, the risk of capsule retention was increased as high as 13.2% [58]. Although no serious adverse events were reported with CCE-1 [46,47] or CCE-2 [49,51] in IBD patients, theoretically, the increased diameter of the CCE induces capsule retention in IBD patients with unrecognized small-bowel strictures [55].
Although CCE has distinct merits, limitations also exist. First, similar to conventional colonoscopy and CTC, CCE requires bowel preparation and prolonged and complicated preparation. The extensive need for laxatives may hinder patient and physician enthusiasm for CCE. One study for bowel preparations for CCE suggests that a 2 L PEG plus ascorbic acid regimen is at least as effective as a 4 L PEG regimen [16,59].
Experimental CCE facilitating image acquisition without bowel preparation has been described, but is impractical [60]. Second, CCE is not yet shown to be cost effective. The average cost of CCE has been estimated at approximately $950 in the United States and €700 in Europe [23]. Third, experienced and trained interpreters of CCE results are not yet widely available. There is a need for experts in CCE to share their experience and train others. Computer-based learning and computer-aided analysis of CCE findings have been reported and may be improved in the future [61]. Finally, similar to other capsule endoscopy, CCE is limited by inability to control movement, insufflate the intestine wall, aspirate liquids, and clean the mucosal surface. Patients with significant polyps are not amenable to biopsy and polypectomy. Subsequent polypectomy will require increased resource utilization.
CCE is a new concept based on the possibility of visualization of the entire gastrointestinal tract from mouth to anus (pan-endoscopy) with a single non-invasive procedure [62]. CCE-2, with its two cameras that record video images from both ends and adaptive frame rate technique, provides detailed images and additional tissue coverage, potentially of the entire gastrointestinal tract. Preliminary studies have demonstrated the feasibility of pan-endoscopy, although other segments (esophagus, stomach) need technical improvements for effective visualization [63,64]. A series of 24 subjects undergoing CCE revealed pathologies of esophagus, stomach, and small intestine in 7, 9, and 14 subjects, respectively [65]. This pan-endoscopic examination facilitates the evaluation of CD and drug effects throughout the gastrointestinal tract [65].
The reading time for CCE is longer than that for small bowel or esophagus capsule endoscopy. Furthermore, the evaluation of entire gastrointestinal tract to determine potential extra-colonic findings take more time than the examination of colon using CCE. However, technological advances based on artificial intelligence and machine learning may support and improve capsule interpretation. Computer-aided lesion detection will significantly reduce the reading time. Future CCE may allow shorter reading time for interpretation in parallel with the development of more efficient software [66].
Pan-endoscopy using CCE may be available. However, the inability of therapeutic capabilities to obtain biopsies and control its locomotion limits the clinical use of CCE. Several previous studies attempted to overcome these limitations and experimental prototypes of a capsule controlled by remote manipulation [67-70], designed for virtual biopsies and drug delivery [71] have been tested. Although the exploration of the esophagus and stomach remain sub-optimal with the currently available CCE models, the feasibility of remotely controlled prototypes suggests successful resolution of such limitations in the near future [68-70,72].
CCE has emerged as a promising new modality for colonic evaluation. The greatest advantage of CCE relates to noninvasive and painless endoscopic colonic examination that does not require sedation. Therefore, CCE may be well-tolerated by patients in an outpatient setting, resulting in increased patient compliance. Patients at increased risk for advanced CRN or CRC, such as those with a history of previous CRN or CRC and alarm symptoms or signs, should be referred to colonoscopy. However, when the patients are contraindicated or unwilling to undergo colonoscopy, CCE may be discussed with the patient as the “next-step” after conventional colonoscopy. Based on preliminary data, CCE may be useful in monitoring inflammation associated with UC, which may facilitate therapy. To date, there is insufficient data to support the use of CCE as a primary CRC screening tool and diagnostic or surveillance modality in patients with IBD. Randomized studies evaluating the efficacy of CCE compared with radiological or conventional endoscopic modalities are needed to confirm the utility of CCE and identify patients who are candidates for CCE. Considering the rapid technological advances, the expansion of CCE in the field of CRC screening and assessment of IBD activity is foreseeable in the near future.
REFERENCES
1. Dominitz JA, Eisen GM, Baron TH, et al. Complications of colonoscopy. Gastrointest Endosc. 2003; 57:441–445.
2. Shafer LA, Walker JR, Waldman C, et al. Factors associated with anxiety about colonoscopy: the preparation, the procedure, and the anticipated findings. Dig Dis Sci. 2018; 63:610–618.

4. Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010; 71:280–286.

5. Eliakim R, Fireman Z, Gralnek IM, et al. Evaluation of the PillCam colon capsule in the detection of colonic pathology: results of the first multicenter, prospective, comparative study. Endoscopy. 2006; 38:963–970.

6. Eliakim R, Yassin K, Niv Y, et al. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy. 2009; 41:1026–1031.

7. Spada C, Hassan C, Galmiche JP, et al. Colon capsule endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2012; 44:527–536.

8. Kim YS, Hong CW, Kim BC, et al. Randomized clinical trial comparing reduced-volume oral picosulfate and a prepackaged low-residue diet with 4-liter PEG solution for bowel preparation. Dis Colon Rectum. 2014; 57:522–528.

9. Spada C, Riccioni ME, Hassan C, Petruzziello L, Cesaro P, Costamagna G. PillCam colon capsule endoscopy: a prospective, randomized trial comparing two regimens of preparation. J Clin Gastroenterol. 2011; 45:119–124.
10. Van Gossum A, Munoz-Navas M, Fernandez-Urien I, et al. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med. 2009; 361:264–270.

11. Schoofs N, Devière J, Van Gossum A. PillCam colon capsule endoscopy compared with colonoscopy for colorectal tumor diagnosis: a prospective pilot study. Endoscopy. 2006; 38:971–977.

12. Spada C, Hassan C, Marmo R, et al. Meta-analysis shows colon capsule endoscopy is effective in detecting colorectal polyps. Clin Gastroenterol Hepatol. 2010; 8:516–522.

13. Garborg K, de Lange T, Bretthauer M. Quality indicators in colonoscopy. Curr Treat Options Gastroenterol. 2017; 15:416–428.

14. Gay G, Delvaux M, Frederic M, Fassler I. Could the colonic capsule PillCam colon be clinically useful for selecting patients who deserve a complete colonoscopy?: results of clinical comparison with colonoscopy in the perspective of colorectal cancer screening. Am J Gastroenterol. 2010; 105:1076–1086.

15. Wexner SD, Beck DE, Baron TH, et al. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Gastrointest Endosc. 2006; 63:894–909.

16. Ramos L, Alarcón O, Adrian Z, et al. One-day versus two-day cleansing for colon capsule endoscopy: a prospective randomized pilot study. Gastroenterol Hepatol. 2014; 37:101–106.

17. Hartmann D, Keuchel M, Philipper M, et al. A pilot study evaluating a new low-volume colon cleansing procedure for capsule colonoscopy. Endoscopy. 2012; 44:482–486.

18. Spada C, Hassan C, Ingrosso M, et al. A new regimen of bowel preparation for PillCam colon capsule endoscopy: a pilot study. Dig Liver Dis. 2011; 43:300–304.

19. Shah HA, Paszat LF, Saskin R, Stukel TA, Rabeneck L. Factors associated with incomplete colonoscopy: a population-based study. Gastroenterology. 2007; 132:2297–2303.

20. Kakugawa Y, Saito Y, Saito S, et al. New reduced volume preparation regimen in colon capsule endoscopy. World J Gastroenterol. 2012; 18:2092–2098.

21. Riccioni ME, Urgesi R, Cianci R, Bizzotto A, Spada C, Costamagna G. Colon capsule endoscopy: advantages, limitations and expectations. Which novelties? World J Gastrointest Endosc. 2012; 4:99–107.

22. Ladas SD, Triantafyllou K, Spada C, et al. European Society of Gastrointestinal Endoscopy (ESGE): recommendations (2009) on clinical use of video capsule endoscopy to investigate small-bowel, esophageal and colonic diseases. Endoscopy. 2010; 42:220–227.

23. Han YM, Im JP. Colon capsule endoscopy: where are we and where are we going. Clin Endosc. 2016; 49:449–453.

24. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2017; 112:1016–1030.

25. Berrington de Gonzalez A, Kim KP, Yee J. CT colonography: perforation rates and potential radiation risks. Gastrointest Endosc Clin N Am. 2010; 20:279–291.
26. Johnson CD, Herman BA, Chen MH, et al. The National CT Colonography Trial: assessment of accuracy in participants 65 years of age and older. Radiology. 2012; 263:401–408.

27. Stoop EM, de Haan MC, de Wijkerslooth TR, et al. Participation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: a randomised controlled trial. Lancet Oncol. 2012; 13:55–64.

28. Sakamoto T, Mitsuzaki K, Utsunomiya D, et al. Detection of flat colorectal polyps at screening CT colonography in comparison with conventional polypoid lesions. Acta Radiol. 2012; 53:714–719.

29. IJspeert JE, Tutein Nolthenius CJ, Kuipers EJ, et al. CT-colonography vs. colonoscopy for detection of high-risk sessile serrated polyps. Am J Gastroenterol. 2016; 111:516–522.

30. Rondonotti E, Borghi C, Mandelli G, et al. Accuracy of capsule colonoscopy and computed tomographic colonography in individuals with positive results from the fecal occult blood test. Clin Gastroenterol Hepatol. 2014; 12:1303–1310.

31. Spada C, Hassan C, Barbaro B, et al. Colon capsule versus CT colonography in patients with incomplete colonoscopy: a prospective, comparative trial. Gut. 2015; 64:272–281.

32. Rex DK, Lieberman DA. A survey of potential adherence to capsule colonoscopy in patients who have accepted or declined conventional colonoscopy. J Clin Gastroenterol. 2012; 46:691–695.

33. Rokkas T, Papaxoinis K, Triantafyllou K, Ladas SD. A meta-analysis evaluating the accuracy of colon capsule endoscopy in detecting colon polyps. Gastrointest Endosc. 2010; 71:792–798.

34. Pilz JB, Portmann S, Peter S, Beglinger C, Degen L. Colon capsule endoscopy compared to conventional colonoscopy under routine screening conditions. BMC Gastroenterol. 2010; 10:66.

35. Sacher-Huvelin S, Coron E, Gaudric M, et al. Colon capsule endoscopy vs. colonoscopy in patients at average or increased risk of colorectal cancer. Aliment Pharmacol Ther. 2010; 32:1145–1153.

36. Brechmann T, Schmiegel W, Klute L, Rösch T, Pox C. Feasibility of a colon capsule overnight procedure followed by colonoscopy. Z Gastroenterol. 2016; 54:146–151.

37. Alvarez-Urturi C, Fernández-Esparrach G, Ibáñez IA, et al. Accuracy of colon capsule endoscopy in detecting colorectal polyps in individuals with familial colorectal cancer: could we avoid colonoscopies? Gastroenterol Res Pract. 2017; 2017:1507914.

38. Spada C, Hassan C, Munoz-Navas M, et al. Second-generation colon capsule endoscopy compared with colonoscopy. Gastrointest Endosc. 2011; 74:581–589. e1.

39. Hagel AF, Gäbele E, Raithel M, et al. Colon capsule endoscopy: detection of colonic polyps compared with conventional colonoscopy and visualization of extracolonic pathologies. Can J Gastroenterol Hepatol. 2014; 28:77–82.

40. Rex DK, Adler SN, Aisenberg J, et al. Accuracy of capsule colonoscopy in detecting colorectal polyps in a screening population. Gastroenterology. 2015; 148:948–957. e2.

41. Saito Y, Saito S, Oka S, et al. Evaluation of the clinical efficacy of colon capsule endoscopy in the detection of lesions of the colon: prospective, multicenter, open study. Gastrointest Endosc. 2015; 82:861–869.

42. Igawa A, Oka S, Tanaka S, Otani I, Kunihara S, Chayama K. Evaluation for the clinical efficacy of colon capsule endoscopy in the detection of laterally spreading tumors. Digestion. 2017; 95:43–48.

43. Ota Y, Yamada A, Kobayashi Y, et al. Diagnostic capability of colon capsule endoscopy for advanced colorectal cancer: a pilot study. Dig Endosc. 2017; 29:695–701.

44. Parodi A, Vanbiervliet G, Hassan C, et al. Colon capsule endoscopy to screen for colorectal neoplasia in those with family histories of colorectal cancer. Gastrointest Endosc. 2018; 87:695–704.
45. Given Imaging’s PillCam® COLON cleared in Japan, world’s second largest healthcare market [Internet]. Yoqneam: Given Imaging Ltd;c2013. [updated 2013 Jul 15; cited 2018 Jul 27]. Available from: https://globenewswire.com/news-release/2013/07/15/559866/10040275/en/Given-Imaging-s-PillCam-R-COLON-Cleared-in-Japan-World-s-Second-Largest-Healthcare-Market.html.
46. Sung J, Ho KY, Chiu HM, Ching J, Travis S, Peled R. The use of Pillcam colon in assessing mucosal inflammation in ulcerative colitis: a multicenter study. Endoscopy. 2012; 44:754–758.

47. Meister T, Heinzow HS, Domagk D, et al. Colon capsule endoscopy versus standard colonoscopy in assessing disease activity of ulcerative colitis: a prospective trial. Tech Coloproctol. 2013; 17:641–646.

48. Ye CA, Gao YJ, Ge ZZ, et al. PillCam colon capsule endoscopy versus conventional colonoscopy for the detection of severity and extent of ulcerative colitis. J Dig Dis. 2013; 14:117–124.
49. Shi HY, Chan FKL, Higashimori A, et al. A prospective study on second-generation colon capsule endoscopy to detect mucosal lesions and disease activity in ulcerative colitis (with video). Gastrointest Endosc. 2017; 86:1139–1146. e6.

50. Oliva S, Di Nardo G, Hassan C, et al. Second-generation colon capsule endoscopy vs. colonoscopy in pediatric ulcerative colitis: a pilot study. Endoscopy. 2014; 46:485–492.

51. D’Haens G, Löwenberg M, Samaan MA, et al. Safety and feasibility of using the second-generation Pillcam colon capsule to assess active colonic Crohn’s disease. Clin Gastroenterol Hepatol. 2015; 13:1480–1486. e3.

52. Oliva S, Cucchiara S, Civitelli F, et al. Colon capsule endoscopy compared with other modalities in the evaluation of pediatric Crohn’s disease of the small bowel and colon. Gastrointest Endosc. 2016; 83:975–983.

53. Niv Y, Gal E, Gabovitz V, Hershkovitz M, Lichtenstein L, Avni I. Capsule Endoscopy Crohn’s Disease Activity Index (CECDAIic or Niv Score) for the small bowel and colon. J Clin Gastroenterol. 2018; 52:45–49.

54. Leighton JA, Helper DJ, Gralnek IM, et al. Comparing diagnostic yield of a novel pan-enteric video capsule endoscope with ileocolonoscopy in patients with active Crohn’s disease: a feasibility study. Gastrointest Endosc. 2017; 85:196–205. e1.

55. Enns RA, Hookey L, Armstrong D, et al. Clinical practice guidelines for the use of video capsule endoscopy. Gastroenterology. 2017; 152:497–514.
56. Applicability of second-generation colon capsule endoscope to ulcerative colitis: a clinical feasibility study. J Gastroenterol Hepatol. 2013; 28:1174–1179.
57. ASGE Standards of Practice Committee, Saltzman JR, Cash BD, et al. Bowel preparation before colonoscopy. Gastrointest Endosc. 2015; 81:781–794.
58. Cheifetz AS, Kornbluth AA, Legnani P, et al. The risk of retention of the capsule endoscope in patients with known or suspected Crohn’s disease. Am J Gastroenterol. 2006; 101:2218–2222.

59. Argüelles-Arias F, San-Juan-Acosta M, Belda A, et al. Preparations for colon capsule endoscopy. Prospective and randomized comparative study between two preparations for colon capsule endoscopy: PEG 2 liters + ascorbic acid versus PEG 4 liters. Rev Esp Enferm Dig. 2014; 106:312–317.
60. Chatrath H, Rex DK. Potential screening benefit of a colorectal imaging capsule that does not require bowel preparation. J Clin Gastroenterol. 2014; 48:52–54.

61. Watabe H, Nakamura T, Yamada A, Kakugawa Y, Nouda S, Terano A. Assessment of an electronic learning system for colon capsule endoscopy: a pilot study. J Gastroenterol. 2016; 51:579–585.

62. Rosa B, Carvalho PB, Cotter J. Pillcam colon 2 in Crohn’s disease: pan-enteric tool for a pan-enteric disease. International Journal of Gastroenterology Disorders & Therapy. 2015; 2:116.

63. Remes-Troche JM, Jiménez-García VA, García-Montes JM, Hergueta-Delgado P, Roesch-Dietlen F, Herrerías-Gutiérrez JM. Application of colon capsule endoscopy (CCE) to evaluate the whole gastrointestinal tract: a comparative study of single-camera and dual-camera analysis. Clin Exp Gastroenterol. 2013; 6:185–192.

64. Triantafyllou K, Beintaris I, Dimitriadis GD. Is there a role for colon capsule endoscopy beyond colorectal cancer screening? A literature review. World J Gastroenterol. 2014; 20:13006–13014.

65. Friedel D, Modayil R, Stavropoulos S. Colon capsule endoscopy: review and perspectives. Gastroenterol Res Pract. 2016; 2016:9643162.

66. Singeap AM, Stanciu C, Trifan A. Capsule endoscopy: the road ahead. World J Gastroenterol. 2016; 22:369–378.

67. Sharma VK. The future is wireless: advances in wireless diagnostic and therapeutic technologies in gastroenterology. Gastroenterology. 2009; 137:434–439.

68. Keller J, Fibbe C, Volke F, et al. Inspection of the human stomach using remote-controlled capsule endoscopy: a feasibility study in healthy volunteers (with videos). Gastrointest Endosc. 2011; 73:22–28.

69. Swain P, Toor A, Volke F, et al. Remote magnetic manipulation of a wireless capsule endoscope in the esophagus and stomach of humans (with videos). Gastrointest Endosc. 2010; 71:1290–1293.
70. Ciuti G, Donlin R, Valdastri P, et al. Robotic versus manual control in magnetic steering of an endoscopic capsule. Endoscopy. 2010; 42:148–152.

71. Schostek S, Schurr MO. European research on wireless endoscopy--the VECTOR project. Stud Health Technol Inform. 2013; 189:193–199.
Fig. 1.
Colon capsule endoscopy (Given Imaging Ltd., Yoqneam, Israel). (A) Upper panel represents the first generation colon capsule endoscopy (CCE-1) and the lower portion is the second generation colon capsule endoscopy (CCE-2). CCE-2 is slightly bigger than CCE-1. (B) Acetaminophen 650 mg (Tyrenol SR®) and CCE-2. (C) CCE-2 measures 11.6×31.5 mm, equal to 1 and 1/2 of the last joint of finger in an adult male.
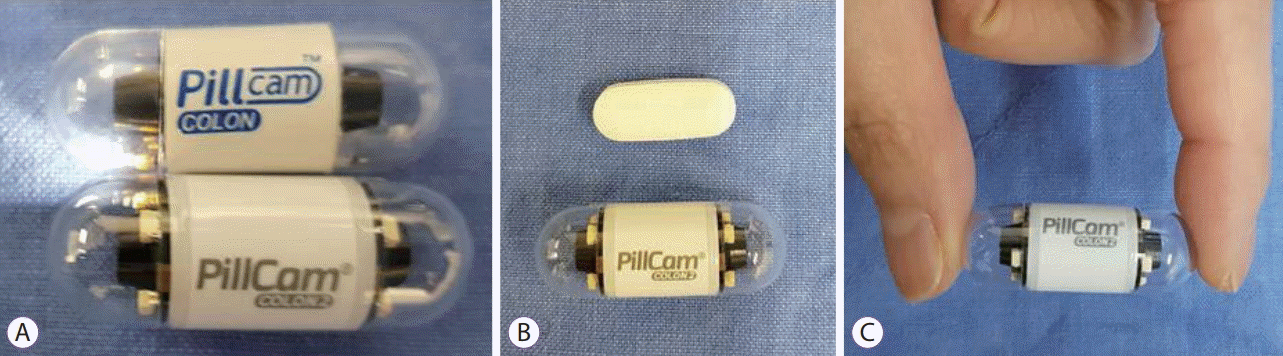
Fig. 2.
Examples of endoscopic pictures captured by colon capsule endoscopy (CCE). (A) Normal colonic mucosa. (B) Colorectal neoplasm: 17 mm-sized polyp was found at transverse colon during screening CCE (Courtesy of Professor Jae Jun Park from Yonsei University College of Medicine, Seoul, Korea).
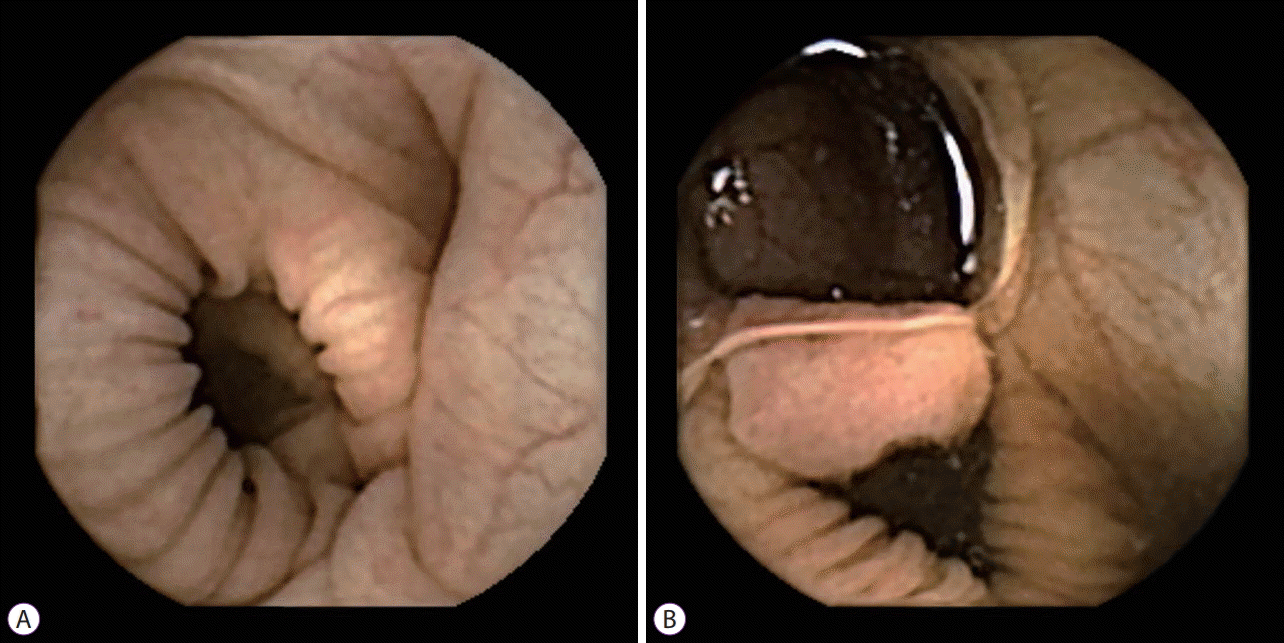
Table 1.
Table 2.
Sensitivity and Specificity of Colon Capsule Endoscopy for Polyp Detection
| Study | Type of colon capsule | No. of patients | Outcome measurement | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Eliakim et al. (2006) [5] | CCE-1 | 84 | Polyps ≥ 6 mm in size or ≥3 in number, Per-patient analysis | 50 | 83 |
| Schoofs et al. (2006) [11] | CCE-1 | 36 | Polyps ≥ 6 mm in size or ≥3 in number, Per-patient analysis | 77 | 70 |
| Van Gossum et al. (2009) [10] | CCE-1 | 320 | Polyp ≥6 mm, Per-patient analysis | 64 | 84 |
| Advanced adenoma ≥6 mm, Per-patient analysis | 73 | 79 | |||
| Polyp ≥6 mm, Per-patient analysis | 74 | 74 | |||
| Eliakim et al. (2009) [6] | CCE-2 | 98 | Any polyp, Per-patient analysis | 44 | 53 |
| Polyp ≥6 mm, Per-patient analysis | 89 | 76 | |||
| Polyp ≥10 mm, Per-patient analysis | 88 | 89 | |||
| Gay et al. (2010) [14] | CCE-1 | 126 | Colonoscopy results | 87.5 | 76 |
| Sacher-Huvelin et al. (2010) [35] | CCE-1 | 545 | Polyp ≥6 mm, Per-patient analysis | 39 | 88 |
| Spada et al. (2011) [38] | CCE-2 | 109 | Polyp ≥6 mm, Per-patient analysis | 84 | 64 |
| Rondonotti et al. (2014) [30] | CCE-2 | 50 | Polyp ≥6 mm, Per-patient analysis, | 88.2 | 87.8 |
| Rex et al. (2015) [40] | CCE-2 | 695 | Polyp ≥6 mm, Per-patient analysis | 81 | 93 |
| Polyp ≥10 mm, Per-patient analysis | 80 | 97 | |||
| Saito et al. (2015) [41] | CCE-2 | 66 | Polyp ≥6 mm or any other lesion warranting endoscopic or surgical treatment | ||
| Per-patient analysis | 94.0 | - | |||
| Per-polyp analysis | 86.6 | - | |||
| Brechmann et al. (2016) [36] | CCE-1 | 50 | Any polyps, Per-polyp analysis | 65 | 76 |
| Igawa et al. (2017) [42] | CCE-2 | 30 | Laterally spreading tumors Per-polyp analysis | 81 | 100 |
| Alvarez-Urturi et al. (2017) [37] | CCE-1 | 53 | Advanced adenomas, Per-polyp analysis | 100 | 98 |
| Any polyp, Per-patient analysis | 87 | 97 | |||
| Ota et al. (2017) [43] | CCE-2 | 21 | Advanced colorectal cancer | ||
| Per-patient analysis | 85 | - | |||
| Per-polyp analysis | 81 | - | |||
| Parodi et al. (2018) [44] | CCE-2 | 177 | Any polyp ≥6 mm, Per-patient analysis | 91 | 95 |
| Any polyp ≥10 mm, Per-patient analysis | 89 | 95 |
Table 3.
Outcome of Colon Capsule Endoscopy Compared with Ileo-Colonoscopy in Patients with Inflammatory Bowel Diseases
| Study | Type of colon capsule | No. of patients | Outcome measurement | Results of CCE |
|---|---|---|---|---|
| Ulcerative colitis | ||||
| Sung et al. (2012) [46] | CCE-1 | 96 | Colonic inflammation (defined as the presence of ulcers, erythema, erosions, edema, exudates in mucosa) | Sensitivity, 89% (95% CI, 80–95); |
| Specificity, 75% (95% CI, 51–90); | ||||
| PPV, 93% (95% CI, 84–97); | ||||
| NPV, 65% (95% CI, 43–83) | ||||
| Meister et al. (2013) [47] | CCE-1 | 13 | Modified Rachmilewitz score | Colonoscopy group: 7.3±2.9 |
| CCE group: 4.8±3.4 | ||||
| Ye et al. (2013) [48] | CCE-1 | 26 | Extent of mucosal damage, inflammatory lesions | Correlation: severity (κ=0.751, p<0.001), extent (κ=0.522, p<0.001) |
| Hosoe et al. (2013) [56] | CCE-2 | 40 | Matts score | Strong correlation (average rho = 0.797) |
| Oliva et al. (2014) [50] | CCE-2 | 30 (pediatric ulcerative colitis) | Modified Matts score | Sensitivity, 96% (95% CI, 79–99); |
| Specificity, 100% (95% CI, 85–100); | ||||
| PPV, 100% (95% CI, 85–100); | ||||
| NPV, 85% (95% CI, 49–97) | ||||
| Shi et al. (2017) [49] | CCE-2 | 108 | Mayo endoscopic subscore, UCEIS | Per-patient analysis: ICC for Mayo endoscopic subscore, 0.69 (95% CI, 0.46–0.81), ICC for UCEIS, 0.64 (95% CI, 0.38–0.78) |
|
|
||||
| Crohn’s disease | ||||
| D’Haens et al. (2015) [51] | CCE-2 | 40 | CDEIS | ICC of CDEIS, 0.65 (95% CI, 0.43–0.80) |
| Oliva et al. (2016) [52] | CCE-2 | 38 | Active inflammation: colon, CDEIS >3; small bowel, Lew-is score ≥135) | Colon: sensitivity, 89%, specificity, 100%; PPV, 100%; NPV, 91%, Small bowel: sensitivity, 90%, specificity, 94%, PPV, 95%; NPV, 90%, entire GI tract: sensitivity, 89%, specificity, 92%, PPV, 96%; NPV, 79% |
| Niv et al. (2018) [53] | CCE-2 | 10 | Capsule endoscopy Crohn’s disease activity Index | Kendall’s coefficients for the small bowel (0.85, p<0.001) and for the whole intestine (0.77, p<0.001) |
| Leighton et al. (2017) [54] | SBC | 66 | Lesions indicative of active Crohn’s disease (aphthous ulcers, ulcers other than aphthous-type, bleeding, or inflammatory stricture) | Per-patients analysis for diagnostic yield: SBC, 83.3% vs. ileo-colonoscopy, 69.7%; active lesions were detected in both (n=43), SBC only (n=12), and ileocolonoscopy only (n=3) |
CCE-1, first generation colon capsule endoscopy; CCE-2, second generation colon capsule endoscopy; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; ICC, intraclass correlation coefficient; UCEIS, ulcerative colitis endoscopic index of severity; CDEIS, Crohn’s disease endoscopic index of severity; GI, gastrointestinal; SBC, small-bowel colon capsule.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download