INTRODUCTION
MATERIALS AND METHODS
RESULTS
DISCUSSION
Patient characteristics
Anatomy and indication
Prior interventions
Procedure characteristics
Technique
1. Incomplete occlusion:
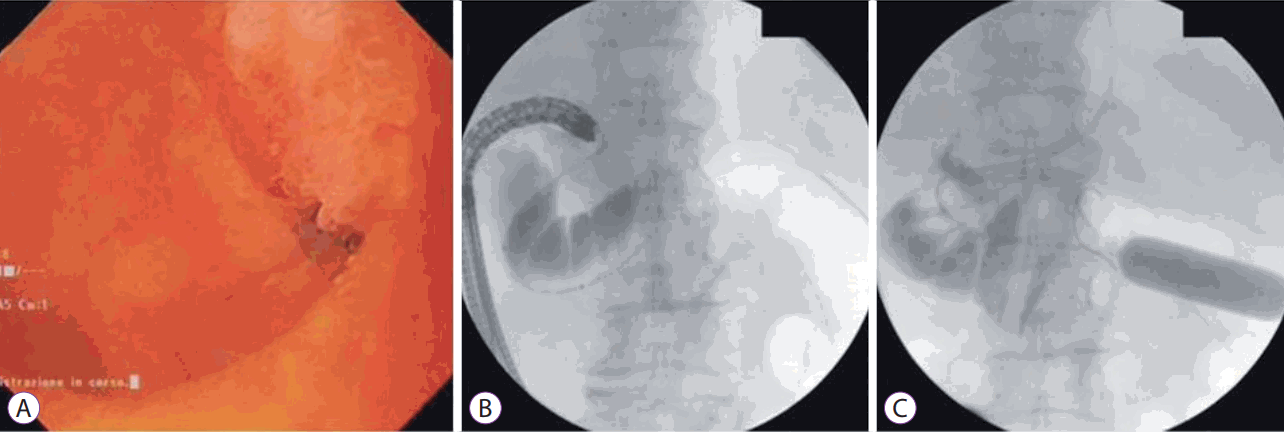
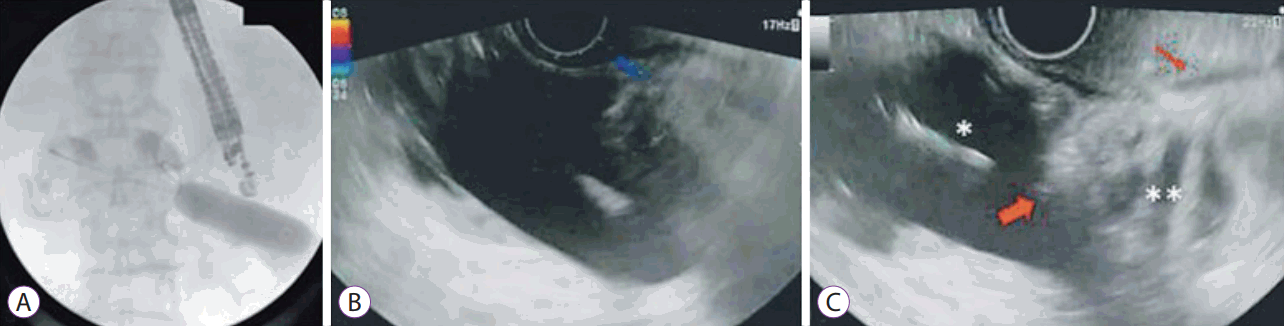
• Balloon or nasobiliary drain assisted: [11,13,14,19,23,24] In this approach, the area of stenosis is traversed either by the endoscope itself or with a guidewire under fluoroscopic guidance (Fig. 1). A balloon dilator or nasobiliary drain is then passed over the guidewire and the balloon is filled with contrast (or the nasobiliary drain is used to insufflate the lumen with water), which can then be localized by an echoendoscope in the stomach (Fig. 2).
• Hybrid rendezvous:11 In this approach, the area of stenosis is traversed by an ultra-thin scope, followed by water insufflation of the distal lumen, which can then be identified by an echoendoscope in the stomach.
• EUS-guided balloon occluded gastro-jejunostomy bypass (EPASS): [14] In this approach, the area of stenosis is first traversed with a guidewire over which a special double balloon enteric tube is passed (Tokyo Medical University Type; Create Medic Co., Yokohama, Japan). The two balloons are then inflated with contrast, followed by saline infusion between the two balloons, which holds the small bowel in close proximity to the gastric lumen and allows easy identification by the echoendoscope.
2. Complete or incomplete occlusion:
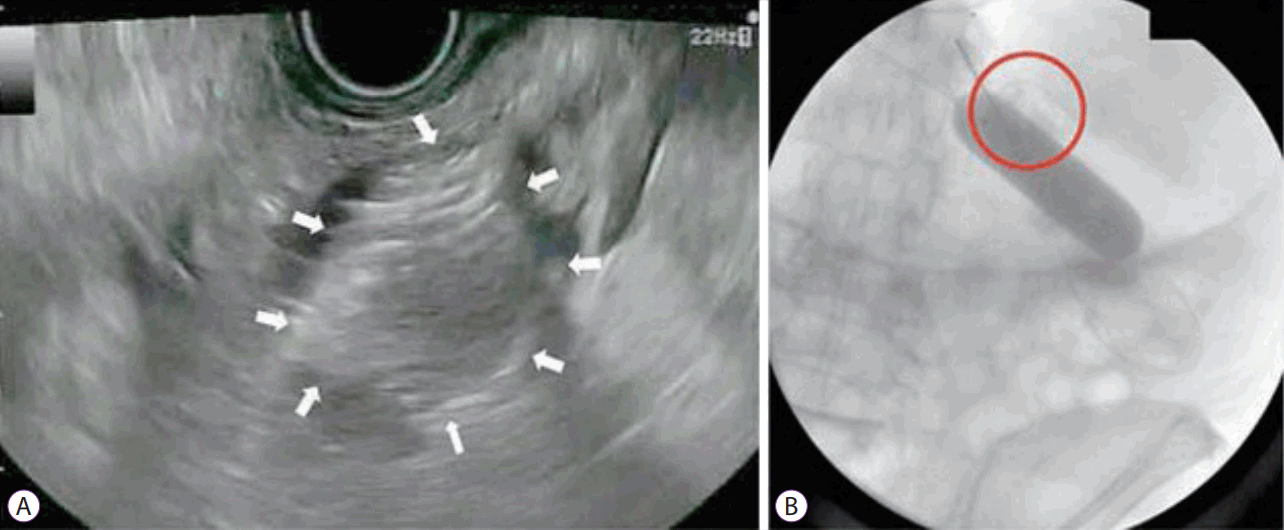
• Direct technique: [11,13,14,16,17,19-24] This approach is feasible even in cases where complete lumen obstruction prevents traversing of the site with a scope or a guidewire. The target small bowel loop is identified and confirmed by contrast injection with the help of EUS-guided needle puncture (19 or 22 G).
• Natural orifice transluminal endoscopic surgery (NOTES): [11] This approach is feasible even in cases with complete luminal occlusion. Essentially, in this approach, a full thickness gastric wall incision is made followed by dilation to allow the endoscope to enter the peritoneal cavity, where under direct visualization, a small bowel loop is identified, incised, and a guidewire is placed, over which the stent is deployed. This approach does not require an echoendoscope.
• Glucagon administration to decrease peristaltic movements
• Snare-balloon technique- A snare is attached over the balloon catheter and is used to catch the guidewire passed through the EUS needle. This is followed by application of tension on the snare/balloon apparatus which helps to keep the target bowel loop fixed in place [13]. A modification of this technique was described by Ngamruengphong et al. where both ends of the guidewire are pulled (externally) to achieve the same outcome [18].




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download