Abstract
Background/Aims
To determine the accuracy of identifying ≥6-mm adenomatous polyps during colonoscopy and define its impact on subsequent interval screening.
Methods
We conducted a retrospective study of patients who underwent colonoscopies at Banner University Medical Center, Tucson from 2011 to 2015. All patients with ≥6-mm adenomatous polyps based on their colonoscopy report were included. Adenomatous polyps were excluded if they did not meet the criteria. Discrepancies in the polyp size were determined by calculating the percentage of size variation (SV). Clinical mis-sizing was defined as SV >33%.
Results
The polyps analyzed were predominantly <10 mm in size. Approximately 13% of the examined polyps met the inclusion criteria, and 40.7% of the adenomas were ≥10 mm. A total of 189 ≥6-mm adenomatous polyps were collected from 10 different gastroenterologists and a colorectal surgeon. Adenomatous polyps were clinically mis-sized in 56.6% of cases and overestimated in 71.4%. Among the adenomas reviewed, 22% of mis-sized polyps and 11% of non-mis-sized polyps resulted in an inappropriate surveillance interval.
After screening for colorectal cancer with colonoscopy, the time interval for subsequent surveillance is determined by polyp histology, number, and size [1-3]. Generally, polyp size is estimated by endoscopists in real-time during colonoscopy. Since adenomatous polyps ≥10 mm in size carry a higher risk of developing into adenocarcinoma, a shorter surveillance interval is recommended [4]. Therefore, the timing of appropriate subsequent colonoscopy surveillance is based on the accurate estimation of polyp size during colonoscopy.
In clinical practice, there is a high degree of subjectivity in estimating polyp size among endoscopists [5-7]. Eichenseer et al. reported that 62% of polyps were incorrectly appraised, with a tendency to overestimate polyp size during endoscopy [6]. As a result, they showed that 35% of patients experienced an inappropriate surveillance interval. However, that study only examined polyps 10–25 mm in size. Therefore, patients with adenomas that were measured as ≥10 mm but that were estimated to be <10 mm by the endoscopist were not analyzed. Several studies have shown a tendency for endoscopists to underestimate polyp size [8-10]. Fennerty et al. found that the polyp size was underestimated in 20% of patients [8]. We have previously shown that only 4% of patients with <6-mm polyps have an inappropriate colonoscopy interval based on polyp mis-sizing and that clinically relevant overestimation of diminutive polyps is rare [11]. While polyps sized <10 mm have been included in other analyses [12], no study has specifically included small polyps (i.e., 6–9 mm), which, when compared to diminutive polyps (i.e., 1–5 mm), may be associated with higher rates of inappropriate colonoscopy intervals. Therefore, owing to the potential for polyp size underestimation, previous studies may have underestimated the degree to which endoscopists’ colorectal cancer surveillance recommendations are impacted by the exclusion of small polyps.
Inaccurate assessment of adenomatous polyp size may lead to more frequent surveillance colonoscopies, exposing patients to undue risk and generating a significant cost. Colonoscopy may be associated with serious complications including cardiopulmonary events, perforation, and hemorrhage. Moreover, unnecessary colonoscopies have significant financial implications. In 2010, the Centers for Medicare and Medicaid Services spent a total of $5.4 billion on colorectal cancer screening, $2.7 billion of which was spent on colonoscopy [13]. Despite these figures, more than a third of the population has not undergone appropriate colorectal cancer screening [14].
In our study, we examined the accuracy among endoscopists in the estimation of small adenomatous polyps and evaluated the impact of mis-sizing on colonoscopy surveillance intervals.
This was a retrospective study examining all outpatient and inpatient colonoscopies performed at the two main campuses of Banner University Medical Center-Tucson from January 1, 2011 through April 30, 2015. The colonoscopies were performed by 10 attending gastroenterologists and one attending colorectal surgeon. Colonoscopy and pathology reports were obtained from the electronic medical records. All endoscopically measured adenomatous polyps ≥6 mm in size were included in the study along with the endoscopy year, patient gender and age, bowel preparation, time of day of the procedure, polyp shape, polyp histology, type of sedation, polypectomy technique, and withdrawal time. The Paris endoscopic classification was used to distinguish polyps by shape [15]. Adenomatous polyps were excluded if they were resected in a piecemeal fashion, incompletely recovered, if multiple fragments of polyp tissue were found in the same specimen jar, or if the endoscopy report did not specify the polyp size. To assign one polyp to one encounter, patients with multiple adenomas were randomized, and one polyp was selected for statistical analysis (Fig. 1).
The study was approved by the Institutional Review Board (IRB No. 1505883454) at the University of Arizona and Banner University Medical Center in Tucson.
Polyps collected at the time of colonoscopy were immediately fixed in formalin. The specimen sat for 8–48 hours before being examined. Pathologic specimens were measured with a millimeter ruler post-fixation.
Discrepancies in the polyp size were determined by calculating the percentage of size variation (SV), defined as: (Endoscopic estimate – Pathology measurement) / (Pathology measurement).
Clinical mis-sizing was defined as SV >33%, in accordance with previous studies [6]. Polyps were deemed to have an inappropriate surveillance recommendation if the endoscopy and pathology report differed in their categorization of advanced adenoma size (≥10 mm) versus non-advanced adenoma size (<10 mm). In patients undergoing polypectomy, decisions regarding the interval of surveillance colonoscopy were made after clinicians received the pathology report describing the polyp histology.
To evaluate for factors that potentially contribute to endoscopic mis-sizing, adenomatous polyps were divided into two groups: (1) clinically mis-sized adenomas (SV >33%) and (2) adenomas that do not meet the definition of mis-sizing (SV ≤33%). All statistical analyses were done using the StataTM 14 software program. One-way ANOVA analysis was used to compare the mis-sizing rate in different years. The Wilcoxon rank-sum analysis was used for mean age, mean polyp size, and quality of bowel preparation. Fisher’s exact test was used to analyze gender, time of day the colonoscopy was performed (i.e., morning vs. afternoon), polyp shape, sedation type, polypectomy technique used, and appropriateness of surveillance recommendations. A p-value <0.05 was considered statistically significant.
Polyps collected from 2011–2015 were predominantly <10 mm and consistent through the years (Fig. 2). The mean mis-sizing rate per year was 41%, and there was no statistical significance between the years. During the study period, 4,827 polyps from 2,938 colonoscopies were reviewed (Fig. 1). Among a total of 270 adenomatous polyps ≥6 mm in size, 81 duplicated polyps were excluded through a randomization method. Approximately 13% of the examined polyps met the inclusion criteria, and 40.7% of the adenomas were ≥10 mm. The histologic distribution of polyps included for analysis was as follows: 157 (83%) tubular, 17 (9%) tubulovillous, and 15 (8%) serrated adenomas.
The mean age of patients was 62.7 years, and 60.4% were males (Table 1). EC-530HL (Fuji, Valhalla, NY, USA) and CF 160 (Olympus, Center Valley, PA, USA) colonoscopes were used in this study. The average endoscopic estimate size and pathologic measurement was 10 and 7.6 mm, respectively. Sixty percent of the included polyps were removed with snare cautery, 23% were removed using a cold snare, and 11% were removed via cold forceps. Of the included colonoscopies, 67% had a bowel preparation that was rated good or better. The average withdrawal time was 15 minutes.
When comparing mis-sized versus non-mis-sized adenomas, the former was more likely to be removed by cold forceps and cold snare (Table 1). Non-mis-sized polyps were typically removed using a hot snare. There was a higher percentage of sessile polyps in the mis-sized group, but this difference was not statistically significant (57% vs. 39.0%, p=0.063). There were no differences in the other tested variables between the two groups.
Adenomatous polyps were clinically mis-sized in 56.6% of cases, and there was considerable variation in the sizing of polyps among endoscopists (Figs. 3, 4). Eighteen percent of all adenomas reviewed resulted in inappropriate surveillance recommendations. Weighted statistical analysis revealed that endoscopist A had a significant impact on the findings. When endoscopist A was removed from the analysis, 48% of adenomas were clinically mis-sized, resulting in 15% of cases undergoing inappropriate subsequent surveillance. The mean time from fellowship completion was 18.6 years. The rate of inappropriate surveillance recommendations did not differ between endoscopists with ≥5 years of experience following training versus those with less experience (experienced =28, less experienced =5, p=0.36).
Accurate endoscopic polyp measurement is important in determining colorectal cancer risk and recommendations for subsequent colonoscopy intervals. Our findings suggest that 56.6% of adenomatous polyps ≥6 mm were mis-sized by endoscopists, leading 18% of patients to experience inappropriate intervals of colorectal cancer screening.
Assessment of polyp size in vivo during colonoscopy can be challenging. In clinical practice, polyp size can be approximated via: (1) visual estimation, (2) comparison of the polyp size with open biopsy forceps that span a known length, or (3) the use of measuring tools. Endoscopic examination is the most commonly used methodology, but it is highly variable and prone to errors owing to dependence on the observer’s expertise, the lack of a size reference for comparison, and optical distortion from the lighting source and the type of camera lens. This technique has an error range of 6%–62.6% according to previous studies depending on the size of the included polyps and the definition of mis-sized polyps [6,16,17]. Interestingly, although the estimation of polyp size using a direct comparison with biopsy forceps yields fewer inter-observer differences, it does not always lead to lower error rates [16,17]. The discrepancy may be due to the difficulty in aligning the forceps along the largest diameter of the polyp and the limitations in forceps length and shape. Other measuring devices including a calibrated hood and probe also have been utilized to improve the accuracy of polyp size estimation, with the latter measuring polyp size within 5% of the post-polypectomy specimen size [16,18]. The linear probe, with markings on the distal flexible tip, is advanced through the endoscope and aligned against the largest diameter of the polyp for measurement. Although these methods may improve the accuracy of polyp size measurements, the additional time and tedious nature of their use has prevented routine adoption into clinical practice.
Technological advancements have vastly improved the visualization of polyps. High definition scope systems with or without narrow band imaging have been shown to enhance adenoma detection rates, but do not impact polyp size assessment [19]. While other colorectal cancer screening methods like computed tomography colonography have been proposed to address the importance of accurate polyp size measurement, this technology has been associated with error and variability in its estimations [20].
Polyp shape may be an important determinant of the accuracy of polyp size assessment. In one study, the estimated size of non-pedunculated polyps was significantly more likely to be overestimated [12]. In the 2013 CARE study, Pohl et al. reported 31% of sessile adenomas were incompletely resected [21]. Our findings suggest that when compared to pedunculated polyps, sessile polyps have an increased tendency to have their size mis-approximated. The reasons for this may be multifactorial including incomplete polyp resection and the difficulty of distinguishing the borders of sessile lesions [21]. In addition, sessile lesions may be difficult to detect due to their flat and pale appearance and their propensity for being located in the right colon [22].
Evidence suggests that endoscopic polyp estimates do not correlate well with histopathological measurements and can result in inappropriate surveillance intervals [7]. Previous reports have shown that erroneous endoscopic sizing is common among endoscopists with a tendency towards overestimation [6,12,23]. Anderson et al. examined all polyps and found that during colonoscopy, the size of 46% of ≥10 mm polyps was overestimated as compared to the pathology-based assessment of polyp size [12]. They found that many of the polyps estimated to be ≥10 mm during endoscopy were actually <10 mm on pathology measurement; hence, about 50% of these patients had an inappropriately early colonoscopy interval. Another study examining 10–25-mm polyps reported that the size of 61% of polyps was overestimated, resulting in inappropriate surveillance in 47.8% of patients [6]. Our current study shows that 56.6% of polyps have >33% SV and that among these patients, 22.4% had inappropriate surveillance intervals. Importantly, our results were driven by endoscopist polyp size overestimation, as opposed to underestimation. The discrepancy between our results and previous analyses may be explained in part by the inclusion of small polyps in our study. Our data showed that the median polyp size in the mis-sized and non-mis-sized groups was 8 and 10 mm, respectively. In our study, when analyzing only adenomatous polyps ≥10 mm in size, we determined that 43% of polyps were associated with inappropriate surveillance, which is similar to previously reported rates [6].
This study has several strengths. We examined only ≥6-mm adenomatous polyps, which is a group of polyps that are likely to be overestimated and lead to early interval surveillance, but that has not been previously evaluated. In addition, rather than including polypectomies up to one year [6,12], we evaluated polyps over more than four years and showed that our results were consistent over time. Third, unlike several previous studies [6,10,24], we considered endoscopic, patient, and polyp histology factors.
Our results are from a single tertiary center and may not be applicable to all populations. However, our findings are consistent with multiple other studies showing a tendency for polyp size overestimation [6,10,12]. The present study did not account for the effect of formalin fixation and the possibility of post-polypectomy sheering. However, several studies have demonstrated no significant difference in polyp size in comparisons of fresh and fixed specimens [24,25]. Additionally, 87% of polyps were excluded in this study, mostly because they were not associated with a size (Fig. 1). This is consistent with previous studies that examined adenomatous polyps [6]. The possibility of post-polypectomy sheering causing polyp shrinkage may also exist, but previous studies suggest that the polyp diameter changes by only 0.2 mm after resection [25-27]. Finally, there is a risk of incomplete polyp removal and associated underestimation of adenoma size. However, among polyps removed by snare, only those with clear margins were included in the study. In addition, a comparable proportion of incompletely resected polyps in both the mis-sized and non-mis-sized groups can be expected.
Accurate polyp size assessment is important for determining the interval of surveillance colonoscopy. Despite improvements in technology, there is wide variability in polyp size measurement with a tendency towards overestimation among providers. Our results suggest that the measured polyp size from the pathology report after resection should be used to determine the appropriate timing of subsequent surveillance colonoscopy. Further studies are warranted to corroborate these findings.
REFERENCES
1. Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US multi-society task force on colorectal cancer. Gastroenterology. 2012; 143:844–857.

2. Tanaka S, Saitoh Y, Matsuda T, et al. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol. 2015; 50:252–260.

3. Matsuda T, Chiu HM, Sano Y, Fujii T, Ono A, Saito Y. Surveillance colonoscopy after endoscopic treatment for colorectal neoplasia: from the standpoint of the Asia-Pacific region. Dig Endosc. 2016; 28:342–347.

4. Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US multi-society task force on colorectal cancer and the American cancer society. CA Cancer J Clin. 2006; 56:143–159. quiz 184-185.

5. Schachschal G, Mayr M, Treszl A, et al. Endoscopic versus histological characterisation of polyps during screening colonoscopy. Gut. 2014; 63:458–465.

6. Eichenseer PJ, Dhanekula R, Jakate S, Mobarhan S, Melson JE. Endoscopic mis-sizing of polyps changes colorectal cancer surveillance recommendations. Dis Colon Rectum. 2013; 56:315–321.

7. Moug SJ, Vernall N, Saldanha J, McGregor JR, Balsitis M, Diament RH. Endoscopists’ estimation of size should not determine surveillance of colonic polyps. Colorectal Dis. 2010; 12:646–650.

8. Fennerty MB, Davidson J, Emerson SS, Sampliner RE, Hixson LJ, Garewal HS. Are endoscopic measurements of colonic polyps reliable? Am J Gastroenterol. 1993; 88:496–500.
9. Margulies C, Krevsky B, Catalano MF. How accurate are endoscopic estimates of size? Gastrointest Endosc. 1994; 40(2 Pt 1):174–177.

10. Chaptini L, Chaaya A, Depalma F, Hunter K, Peikin S, Laine L. Variation in polyp size estimation among endoscopists and impact on surveillance intervals. Gastrointest Endosc. 2014; 80:652–659.

11. Pham T, Taleban S. Mis-sizing of polyps during colonoscopy does not impact colorectal cancer surveillance recommendations. In : American College of Gastroenterology (ACG) 2015 Annual Scientific Meeting and Postgraduate Course; 2015 Oct 16-21; Honolulu (HI), USA.
12. Anderson BW, Smyrk TC, Anderson KS, et al. Endoscopic overestimation of colorectal polyp size. Gastrointest Endosc. 2016; 83:201–208.

13. Goede SL, Kuntz KM, van Ballegooijen M, et al. Cost-savings to medicare from pre-medicare colorectal cancer screening. Med Care. 2015; 53:630–638.

14. Centers for Disease Control and Prevetion. Colorectal Cancer Control Program (CRCCP) [Internet]. Atlanta (GA): Centers for Disease Control and Prevetion;c2017. [updated 2017 Aug 31; cited 2018 May 23]. Available from: https://www.cdc.gov/cancer/crccp/index.htm.
15. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003; 58(6 Suppl):s3–s43.
16. Gopalswamy N, Shenoy VN, Choudhry U, et al. Is in vivo measurement of size of polyps during colonoscopy accurate? Gastrointest Endosc. 1997; 46:497–502.

17. Kim JH, Park SJ, Lee JH, et al. Is forceps more useful than visualization for measurement of colon polyp size? World J Gastroenterol. 2016; 22:3220–3226.

18. Kume K, Watanabe T, Yoshikawa I, Harada M. Endoscopic measurement of polyp size using a novel calibrated hood. Gastroenterol Res Pract. 2014; 2014:714294.

19. Ashktorab H, Etaati F, Rezaeean F, et al. Can optical diagnosis of small colon polyps be accurate? Comparing standard scope without narrow banding to high definition scope with narrow banding. World J Gastroenterol. 2016; 22:6539–6546.

20. Summers RM. Polyp size measurement at CT colonography: what do we know and what do we need to know? Radiology. 2010; 255:707–720.

21. Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013; 144:74–80.e1.

22. Obuch JC, Pigott CM, Ahnen DJ. Sessile serrated polyps: detection, eradication, and prevention of the evil twin. Curr Treat Options Gastroenterol. 2015; 13:156–170.

23. Schoen RE, Gerber LD, Margulies C. The pathologic measurement of polyp size is preferable to the endoscopic estimate. Gastrointest Endosc. 1997; 46:492–496.

24. Levene Y, Hutchinson JM, Tinkler-Hundal E, Quirke P, West NP. The correlation between endoscopic and histopathological measurements in colorectal polyps. Histopathology. 2015; 66:485–490.

25. Morales TG, Sampliner RE, Garewal HS, Fennerty MB, Aickin M. The difference in colon polyp size before and after removal. Gastrointest Endosc. 1996; 43:25–28.

Fig. 1.
Schematic of polyps that met the study criteria. All polyps included in this study were collected at Banner University Medical Center in Tucson, AZ, USA. Of the reviewed polyps, 12.9% had a reported size and 5% met the criteria for statistical analysis.
Fig. 2.
Endoscopic evaluation of polyp size. Consistency of polyp size estimate per year. The mean mis-sizing rate per year is 41%.
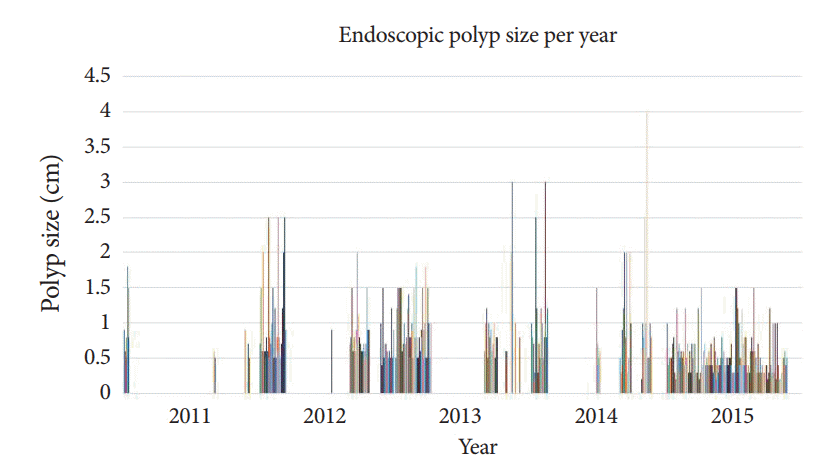
Fig. 3.
Percentage of included polyps with clinical mis-sizing (size variation [SV] >33%) per endoscopist. Six of eleven endoscopists had polyps that were clinically mis-sized. SV is defined as follows: (Endoscopic estimate – Pathology measurement) / (Pathology measurement).
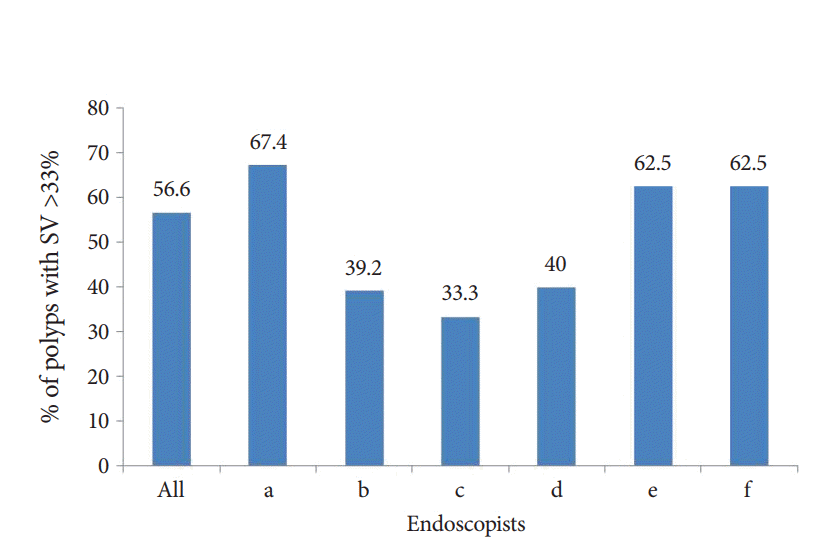
Fig. 4.
Clinical mis-sizing of adenomatous polyps ≥6 mm among different endoscopists. Size variation (SV) is defined as follows: (Endoscopic estimate –Pathology measurement) / (Pathology measurement).
Table 1.
Procedural and Demographic Characteristics of Patients with Adenomatous Polyps ≥6 mm undergoing Colonoscopy




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download