Abstract
The diagnosis and management of pancreatic strictures, whether malignant or benign, remain challenging. The last 2 decades have seen dramatic progress in terms of both advanced imaging and endoscopic therapy. While plastic stents remain the cornerstone of the treatment of benign strictures, the advent of fully covered metal stents has initiated a new wave of interest in calibrating the pancreatic duct with fewer sessions. In malignant disease, palliation remains the priority and further data are necessary before offering systematic pancreatic stenting.
Pancreatic duct stricture is a common problem that is associated with various etiologies. Benign etiologies include chronic pancreatitis, recurrent acute pancreatitis, trauma, surgical complications, and pseudocysts. Pancreatic strictures can also be a manifestation of malignancy [1,2]. The diagnosis and treatment of pancreatic strictures have proven challenging for physicians. Most pancreatic strictures present with persistent or recurrent abdominal pain, or with symptoms of chronic pancreatitis and exocrine insufficiency. The possibility of underlying malignancy necessitates investigation with high-quality cross-sectional imaging including computed tomography and magnetic resonance imaging/magnetic resonance cholangiopancreatography. Any masses that are appreciated on imaging should be followed by endoscopic ultrasonography with fine needle aspiration (EUS-FNA) [3].
EUS-FNA has replaced endoscopic retrograde cholangiopancreatography (ERCP) as the method of choice in obtaining tissue specimen, as it is associated with a higher success rate and fewer post-procedural complications [4]. Pancreatic brushing should be employed in the absence of a definitive mass on imaging [3]. Furthermore, repeat EUS has been shown to improve diagnostic yield in the case of continued suspicion of cancer and to increase the sensitivity of pancreatic mass detection in the setting of chronic pancreatitis [4,5]. Confocal endomicroscopy is another technology that aids in diagnosing neoplasms in indeterminate masses and in the mapping of abnormal pancreatic ducts prior to surgery [6]. Imaging and endoscopic diagnostic tests are ideally correlated with a careful evaluation of the patient’s history to establish the benign or malignant nature of the pancreatic strictures. Serum markers such as IgG4 in autoimmune pancreatitis or CA 19-9 in pancreatic cancer can also be useful in determining the etiology of the stricture.
The treatment of a pancreatic stricture depends on whether it is benign or malignant. Asymptomatic pancreatic strictures can be left without intervention if malignancy has been excluded. Persistent symptoms such as abdominal pain or postprandial pain are the indications for intervention in benign pancreatic strictures. Factors that must be considered when selecting the modality of treatment include the patient’s individual characteristics such as age and comorbidities, the location and number of strictures, and finally, the expertise of the endoscopist in EUS and ERCP. Treatment of pancreatic strictures can be medical, endoscopic, or surgical. Medical treatment of pancreatic duct strictures includes abstinence from alcohol, strict adherence to a low-fat diet, small frequent meals, and pancreatic enzyme supplements to address symptoms of exocrine insufficiency [7].
Rapid advancements in endoscopic technology have put endoscopy at the forefront of the management of pancreatic strictures. The mainstay of endoscopic therapy for pancreatic strictures includes pancreatic sphincterectomy, followed by the dilation of pancreatic stricture and the placement of a pancreatic duct stent (Fig. 1). This sequence is associated with immediate pain relief in 65%–95% of patients and with sustained pain relief in 32%–68% of patients [8,9]. ERCP is also considered the first-line modality in the management of main pancreatic duct obstruction in the setting of chronic pancreatitis [10,11]. Dilation is usually done before stenting and can be performed using wire-guided balloons (4–6 mm), a bougie, or a Soehendra stent retriever [3]. Plastic stents have become the standard endoscopic treatment of main pancreatic duct strictures in the setting of chronic pancreatitis and they are usually left in place for a fixed duration of time or exchanged upon recurrence of symptoms [12,13]. In addition to plastic stents, self-expanding metal stents have also been successfully used in treating pancreatic strictures (Fig. 2); however, plastic stents are associated with more long-term follow-up data and experience [14]. The larger diameter of plastic stents also seems to confer an advantage in terms of the rate of hospitalizations for abdominal pain [15]. A high rate of stricture recurrence upon removal of the stent has been observed, with a 38% recurrence rate at the 2-year follow up and the need for repeat stenting [16]. The number of strictures and the location of the stricture seem to be critical determining factors in the success of endotherapy. A symptomatic chronic pancreatitis patient with main pancreatic duct stricture at the head of the pancreas would be the ideal candidate for ERCP with stenting. Strictures at the tail of pancreas are more difficult to treat endoscopically. Moreover, endoscopic treatment of multiple chronic pancreatitis-related strictures (chain of lakes appearance) is even more challenging [17].
The technical failure rates of ERCP range from 3%–10%. The most common reasons for technical failure include failure of cannulation of the main pancreatic duct, severe strictures, pancreatic stones, or altered anatomy [18-20]. In patients where conventional ERCP failed, EUS-guided drainage of the main pancreatic duct is emerging as a less invasive alternative to surgery. Our team reported a large, international multicenter study on the safety and efficacy of EUS-guided pancreatic drainage (EUS-PD) in patients who failed conventional ERCP therapy for pancreatic strictures. Among a total of 80 patients, technical success was reported in 89% and clinical success was reported in 81% [21]. Initial studies of EUS-PD have demonstrated a wide range of efficacy from 50%–100% [18-20,22,23], which is likely reflective of the inherent learning curve. Conventionally, surgery or percutaneous intervention would be the next step for patients who fail ERCP therapy for pancreatic strictures; however, those approaches are both associated with significant morbidities. Our multicenter, international investigation demonstrated a 20% adverse events rate, which is still significantly lower than the 30% adverse events rate reported with surgical interventions [21,24-26].
Pancreatic cancer is the fifth leading cause of cancer-related deaths in the western world [27]. Pancreatic strictures and main pancreatic ductal obstructions are common manifestations of pancreatic malignancy. The vast majority of pancreatic cancer patients are inoperable at the time of diagnosis and have a very poor prognosis [28]; therefore, the main concern is palliation. As in the case of patients with chronic pancreatitis, endoscopic stenting can be used in the decompression of malignant ductal obstructions that cause postprandial epigastric pain in patients with pancreatic cancer, particularly in patients with tumors in the head of the pancreas [29]. Both plastic and metal stents have been employed in the treatment of malignant pancreatic strictures [12]. Plastic stents have a reported patency of 2 months, which is attributed to their small diameter [30]. On the other hand, metal stents have demonstrated a longer duration of patency, fewer reinterventions, and increased cost-effectiveness in patients with a prognosis longer than 6 months [30,31]. Whether metal or plastic stents provide better results remains to be determined, and further data are also needed to ascertain the efficacy of fully covered self-expanding metal stents in relieving malignant ductal obstructions [30]. With both plastic and metal stents, technical success rates have ranged between 81% and 100%, and rates of pain improvement have ranged between 61% and 100% [12].
The diagnosis and treatment of pancreatic strictures can prove challenging, and a high index of suspicion should always be maintained in approaching patients with pancreatic strictures in order to rule out malignancy as the underlying etiology. High-quality imaging studies, patient history, and serum markers should be obtained in all patients, and the treatment modality of choice depends on the benign or malignant nature of the pancreatic stricture. Benign strictures can be left untreated if they are asymptomatic. Persistent abdominal pain and recurrent attacks of pancreatitis are among the indications for intervention. Patients in whom conventional ERCP endoscopic therapy for benign strictures fails should be offered EUS-PD as a safe, minimally invasive, and effective alternative to surgery, according to the availability of endoscopic expertise. Patients with malignant pancreatic strictures might benefit from palliation provided by the decompression of the pancreatic ductal obstruction, for which both plastic stents (Table 1) and metal stents (Table 2) have been used [10,15,16,32-43]. However, more data and longer follow up periods are needed to establish the superiority of one type of stent over the other. Patients with benign strictures should be followed up in 3- or 6-month intervals if malignancy is suspected.
REFERENCES
1. Catalano MF. Endoscopic treatment of pancreatic duct strictures. Tech Gastrointest Endosc. 1999; 1:168–174.

2. Cohen SA, Siegel JH, Kasmin FE. Treatment of pancreatic strictures. Curr Treat Options Gastroenterol. 2007; 10:347–354.

3. Oza VM, Kahaleh M. Endoscopic management of chronic pancreatitis. World J Gastrointest Endosc. 2013; 5:19–28.

4. Eloubeidi MA, Varadarajulu S, Desai S, Wilcox CM. Value of repeat endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic cancer. J Gastroenterol Hepatol. 2008; 23:567–570.

5. Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005; 62:728–736. ; quiz 751, 753.

6. Kahaleh M, Turner BG, Bezak K, et al. Probe-based confocal LASER endomicroscopy (pCLE) in the pancreatic duct provides direct visualization of ductal structures and AIDS in clinical management. Gastrointest Endosc. 2013; 77(5 Suppl):AB165.
7. Warshaw AL, Banks PA, Fernández-Del Castillo C. AGA technical review: treatment of pain in chronic pancreatitis. Gastroenterology. 1998; 115:765–776.

8. Weber A, Schneider J, Neu B, et al. Endoscopic stent therapy in patients with chronic pancreatitis: a 5-year follow-up study. World J Gastroenterol. 2013; 19:715–720.

9. Talukdar R, Reddy DN. Pancreatic endotherapy for chronic pancreatitis. Gastrointest Endosc Clin N Am. 2015; 25:765–777.

10. Costamagna G, Bulajic M, Tringali A, et al. Multiple stenting of refractory pancreatic duct strictures in severe chronic pancreatitis: long-term results. Endoscopy. 2006; 38:254–259.

11. Dumonceau JM, Devière J, Le Moine O, et al. Endoscopic pancreatic drainage in chronic pancreatitis associated with ductal stones: long-term results. Gastrointest Endosc. 1996; 43:547–555.

12. Mangiavillano B, Pagano N, Baron TH, Luigiano C. Outcome of stenting in biliary and pancreatic benign and malignant diseases: a comprehensive review. World J Gastroenterol. 2015; 21:9038–9054.

13. Gupta R, Reddy DN. Stent selection for both biliary and pancreatic strictures caused by chronic pancreatitis: multiple plastic stents or metallic stents? J Hepatobiliary Pancreat Sci. 2011; 18:636–639.

14. Adler JM, Gardner TB. Endoscopic therapies for chronic pancreatitis. Dig Dis Sci. 2017; 62:1729–1737.

15. Sauer BG, Gurka MJ, Ellen K, Shami VM, Kahaleh M. Effect of pancreatic duct stent diameter on hospitalization in chronic pancreatitis: does size matter? Pancreas. 2009; 38:728–731.
16. Eleftherladis N, Dinu F, Delhaye M, et al. Long-term outcome after pancreatic stenting in severe chronic pancreatitis. Endoscopy. 2005; 37:223–230.

17. Tringali A, Boskoski I, Costamagna G. The role of endoscopy in the therapy of chronic pancreatitis. Best Pract Res Clin Gastroenterol. 2008; 22:145–165.

18. Tessier G, Bories E, Arvanitakis M, et al. EUS-guided pancreatogastrostomy and pancreatobulbostomy for the treatment of pain in patients with pancreatic ductal dilatation inaccessible for transpapillary endoscopic therapy. Gastrointest Endosc. 2007; 65:233–241.

19. François E, Kahaleh M, Giovannini M, Matos C, Devière J. EUS-guided pancreaticogastrostomy. Gastrointest Endosc. 2002; 56:128–133.

20. Brauer BC, Chen YK, Fukami N, Shah RJ. Single-operator EUS-guided cholangiopancreatography for difficult pancreaticobiliary access (with video). Gastrointest Endosc. 2009; 70:471–479.

21. Tyberg A, Sharaiha RZ, Kedia P, et al. EUS-guided pancreatic drainage for pancreatic strictures after failed ERCP: a multicenter international collaborative study. Gastrointest Endosc. 2017; 85:164–169.

22. Vila JJ, Pérez-Miranda M, Vazquez-Sequeiros E, et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: a Spanish national survey. Gastrointest Endosc. 2012; 76:1133–1141.

23. Fujii LL, Topazian MD, Abu Dayyeh BK, et al. EUS-guided pancreatic duct intervention: outcomes of a single tertiary-care referral center experience. Gastrointest Endosc. 2013; 78:854–864.e1.

24. Adams DB, Ford MC, Anderson MC. Outcome after lateral pancreaticojejunostomy for chronic pancreatitis. Ann Surg. 1994; 219:481–487. ; discussion 487-489.

25. Nealon WH, Thompson JC. Progressive loss of pancreatic function in chronic pancreatitis is delayed by main pancreatic duct decompression. A longitudinal prospective analysis of the modified puestow procedure. Ann Surg. 1993; 217:458–466. ; discussion 466-468.

26. Markowitz JS, Rattner DW, Warshaw AL. Failure of symptomatic relief after pancreaticojejunal decompression for chronic pancreatitis. Strategies for salvage. Arch Surg. 1994; 129:374–379. ; discussion 379-380.
27. DiMagno EP, Reber HA, Tempero MA. AGA technical review on the epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma. American gastroenterological association. Gastroenterology. 1999; 117:1464–1484.
28. Wehrmann T, Riphaus A, Frenz MB, Martchenko K, Stergiou N. Endoscopic pancreatic duct stenting for relief of pancreatic cancer pain. Eur J Gastroenterol Hepatol. 2005; 17:1395–1400.

29. Mekaroonkamol P, Willingham FF, Chawla S. Endoscopic management of pain in pancreatic cancer. JOP. 2015; 16:33–40.
30. Sharaiha RZ, Widmer J, Kahaleh M. Palliation of pancreatic ductal obstruction in pancreatic cancer. Gastrointest Endosc Clin N Am. 2013; 23:917–923.

31. Yeoh KG, Zimmerman MJ, Cunningham JT, Cotton PB. Comparative costs of metal versus plastic biliary stent strategies for malignant obstructive jaundice by decision analysis. Gastrointest Endosc. 1999; 49(4 Pt 1):466–471.

32. Cremer M, Devière J, Delhaye M, Baize M, Vandermeeren A. Stenting in severe chronic pancreatitis: results of medium-term follow-up in seventy-six patients. Endoscopy. 1991; 23:171–176.

33. Rösch T, Daniel S, Scholz M, et al. Endoscopic treatment of chronic pancreatitis: a multicenter study of 1000 patients with long-term follow-up. Endoscopy. 2002; 34:765–771.

34. Vitale GC, Cothron K, Vitale EA, et al. Role of pancreatic duct stenting in the treatment of chronic pancreatitis. Surg Endosc. 2004; 18:1431–1434.

35. Weber A, Schneider J, Neu B, et al. Endoscopic stent therapy for patients with chronic pancreatitis: results from a prospective follow-up study. Pancreas. 2007; 34:287–294.
36. Park DH, Kim MH, Moon SH, Lee SS, Seo DW, Lee SK. Feasibility and safety of placement of a newly designed, fully covered self-expandable metal stent for refractory benign pancreatic ductal strictures: a pilot study (with video). Gastrointest Endosc. 2008; 68:1182–1189.

37. Sauer B, Talreja J, Ellen K, Ku J, Shami VM, Kahaleh M. Temporary placement of a fully covered self-expandable metal stent in the pancreatic duct for management of symptomatic refractory chronic pancreatitis: preliminary data (with videos). Gastrointest Endosc. 2008; 68:1173–1178.

38. Moon SH, Kim MH, Park DH, et al. Modified fully covered self-expandable metal stents with antimigration features for benign pancreatic-duct strictures in advanced chronic pancreatitis, with a focus on the safety profile and reducing migration. Gastrointest Endosc. 2010; 72:86–91.

39. Akbar A, Baron TH. Covered self-expanding metal stent use in the pancreatic duct: a case series. Endoscopy. 2012; 44:869–873.

40. Giacino C, Grandval P, Laugier R. Fully covered self-expanding metal stents for refractory pancreatic duct strictures in chronic pancreatitis. Endoscopy. 2012; 44:874–877.

41. Landi R, Tringali A, Bove V, et al. Fully covered self-expandable metal stents to dilate pancreatic duct strictures due to chronic pancreatitis: a pilot study. Gastrointest Endosc. 2016; 83(5 Suppl):AB251–AB252.
Fig. 1.
The use of plastic stents in the treatment of benign pancreatic strictures. (A) Guidewire insertion. (B) Balloon dilation. (C) Plastic stent deployment.
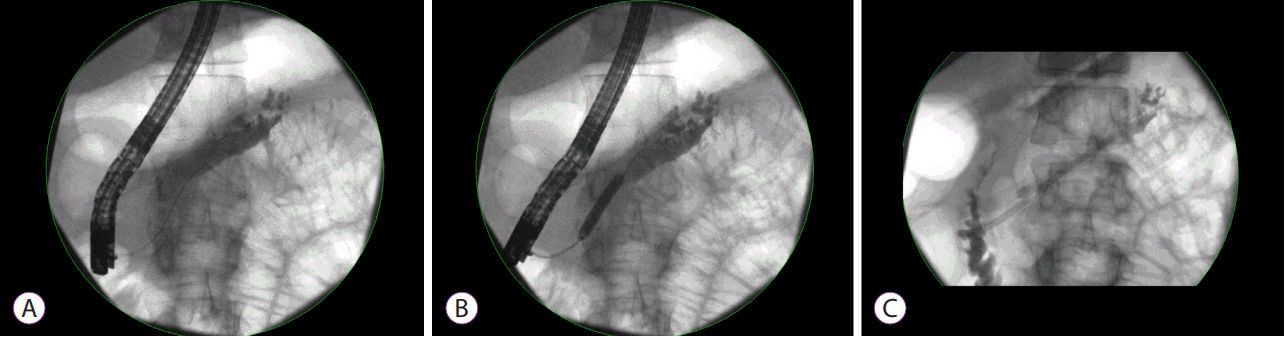
Fig. 2.
The use of fully covered self-expanding metal stent (FCSMS) in treating benign pancreatic strictures. (A) Distal pancreatic stricture identified on fluoroscopy. (B) 8 mm × 60 FCSMS deployment. (C) Removal of the FCSMS at 3 months after deployment. Image showing stricture resolution on pressure injection.
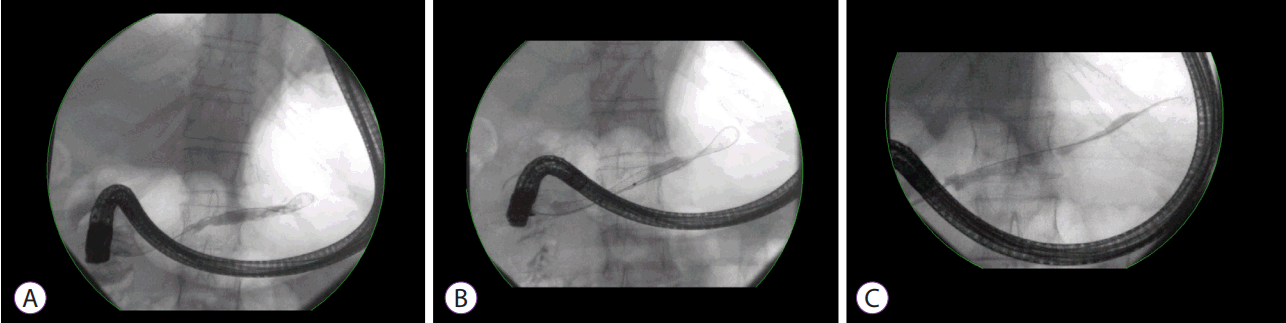
Table 1.
Data on the Use of Plastic Stents in the Treatment of Benign Pancreatic Strictures
| Author | Year | Type of stent | Number of patients | Technical success (%) | Immediate clinical success (%) | Long-term clinical success (%) | Follow-up duration (mo) |
|---|---|---|---|---|---|---|---|
| Cremer et al. [32] | 1991 | Polyethylene | 75 | 98.6 | 94 | 52 | 37 |
| Rösch et al. [33] | 2002 | Various | 478 | 72 | N/A | 63 | 52 |
| Vitale et al. [34] | 2004 | Polyethylene | 89 | 100 | 83 | 63 | 43 |
| Eleftherladis et al. [16] | 2005 | Polyethylene | 100 | 100 | 100 | 70 | 69 |
| Costamagna et al. [10] | 2006 | Polyethylene Amsterdam-type or “Cremer” | 13 | 100 | 100 | 84 | 38 |
| Weber et al. [35] | 2007 | Polyethylene | 17 | 89.4 | 89 | 83 | 24 |
| Sauer et al. [15] | 2009 | Polyethylene | 163 | N/A | N/A | 56 | 36 |
Table 2.
Data on the Use of FCSMS in the Treatment of Benign Pancreatic Strictures
| Author | Year | Type of FCSMS | Number of patients | Technical (%) | Clinical success (%) | Stent placement duration (mo) | Adverse events | Follow-up duration (mo) |
|---|---|---|---|---|---|---|---|---|
| Park et al. [36] | 2008 | Niti-S D-type | 13 | 100 | 100 | 2 | Stent migration (n=5) | 5 |
| Cholestasis (n=2) | ||||||||
| Sauer et al. [37] | 2008 | VIABIL | 6 | 100 | 66 | 3 | None | 8 |
| Moon et al. [38] | 2010 | Niti-S bumpy type | 32 | 100 | 100 | 5 | Stent-induced duct change (n=5) | 20 |
| Mild acute pancreatitis (n=3) | ||||||||
| Akbar et al. [39] | 2012 | VIABIL/WallStent/WallFlex | 9 | 100 | 88.9 | 3.5 | Stent migration (n=1) | 18 |
| Post-procedural abdominal pain (n=1) | ||||||||
| Giacino et al. [40] | 2012 | WallStent/WallFlex | 10 | 100 | 90 | N/A | Post-procedural abdominal pain (n=3) | 19.8 |
| Cholestasis (n=2) | ||||||||
| Landi et al. [41] | 2016 | Nitinol (bumpy stent) | 15 | 100 | 54 | 6 | Cholangitis (n=2) | 18.5 |
| Stent migration (n=2) | ||||||||
| Ogura et al. [42] | 2016 | Niti-S biliary S-type | 13 | 100 | 92 | 5.7 | Stent migration (n=2) | 8.6 |
| Post-procedural abdominal pain (n=1) | ||||||||
| Matsubara et al. [43] | 2016 | Niti-S D-/bumpy type | 10 | 100 | 70% | 3 | Stent-induced duct change (n=2) | 35 |
| Stent migration (n=2) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download