This article has been
cited by other articles in ScienceCentral.
Abstract
Heterotopic pancreas in the stomach is usually asymptomatic and benign. Here, we presented a rare case of an early gastric cancer overlying a heterotopic pancreas. A 48-year-old woman underwent esophagogastroduodenoscopy, which revealed a subepithelial mass measuring 2.0×1.5 cm on the gastric antrum with a 1-cm erosive erythematous discoloration on the surface. A biopsy specimen showed moderately differentiated tubular adenocarcinoma. Endosonography showed a heterogeneous hypoechoic mass measuring 1.3×0.6 cm, with indistinct margins in the second and third layers of the gastric wall; anechoic tubular structures within the mass were suggestive of heterotopic pancreas. Distal gastrectomy was performed, which confirmed an early gastric cancer confined to the mucosa, and a separate underlying heterotopic pancreas. Although heterotopic pancreas is most likely benign, careful endoscopic observation of the mucosal surface is necessary to avoid overlooking a coincident early gastric cancer.
Go to :

Keywords: Early gastric cancer, Heterotopic pancreas, Ectopic pancreas, Endosonography
INTRODUCTION
Heterotopic pancreas, also known as ectopic pancreas, is defined as pancreatic tissue that lacks anatomical and vascular connections to the original pancreas. It can occur anywhere along the gastrointestinal tract; however, in a majority of cases (25%–40%), the ectopic pancreas is found in the stomach [
1]. This condition is usually asymptomatic and is diagnosed incidentally during endoscopy, surgery, or autopsy. Although complications are uncommon, ulceration, bleeding, and obstruction have been observed. Malignant transformation is also rare, although several reports have documented adenocarcinomas arising from heterotopic pancreas.
Meanwhile, an early gastric cancer above a heterotopic pancreas is extremely rare. Here, we report a case of an early gastric cancer combined with an underlying heterotopic pancreas.
Go to :

CASE REPORT
A 48-year-old woman was admitted to our hospital owing to postprandial dyspepsia for several months. She had undergone an appendectomy 30 years previously and a cesarean section 25 years prior. Her vital signs were normal, and physical examination was unremarkable. Laboratory findings were normal. Serum tumor markers including carcinoembryonic antigen (1.38 ng/mL) and cancer antigen 19-9 (7.51 U/mL) were within normal levels.
Esophagogastroduodenoscopy revealed a subepithelial mass measuring 2.0×1.5 cm on the posterior wall of the distal antrum (
Fig. 1A), and a 1-cm erythematous discoloration with erosion on the surface of the mass (
Fig. 1B). A biopsy specimen showed moderately differentiated tubular adenocarcinoma. On endoscopic ultrasonography, a 1.3×0.6-cm heterogeneously hypoechoic mass with indistinct margins was observed to be located in the second and third layers of the gastric wall, and anechoic cystic or tubular structures were observed within the mass (
Fig. 1C,
D), which were suggestive of heterotopic pancreas. Although the carcinoma appeared to be confined to the gastric mucosa, an association between the carcinoma and the underlying heterotopic pancreas was difficult to verify. Abdominal computed tomography showed a protruding mass measuring 1.4×1.0 cm at the distal antrum without lymph node or distant metastases (
Fig. 2).
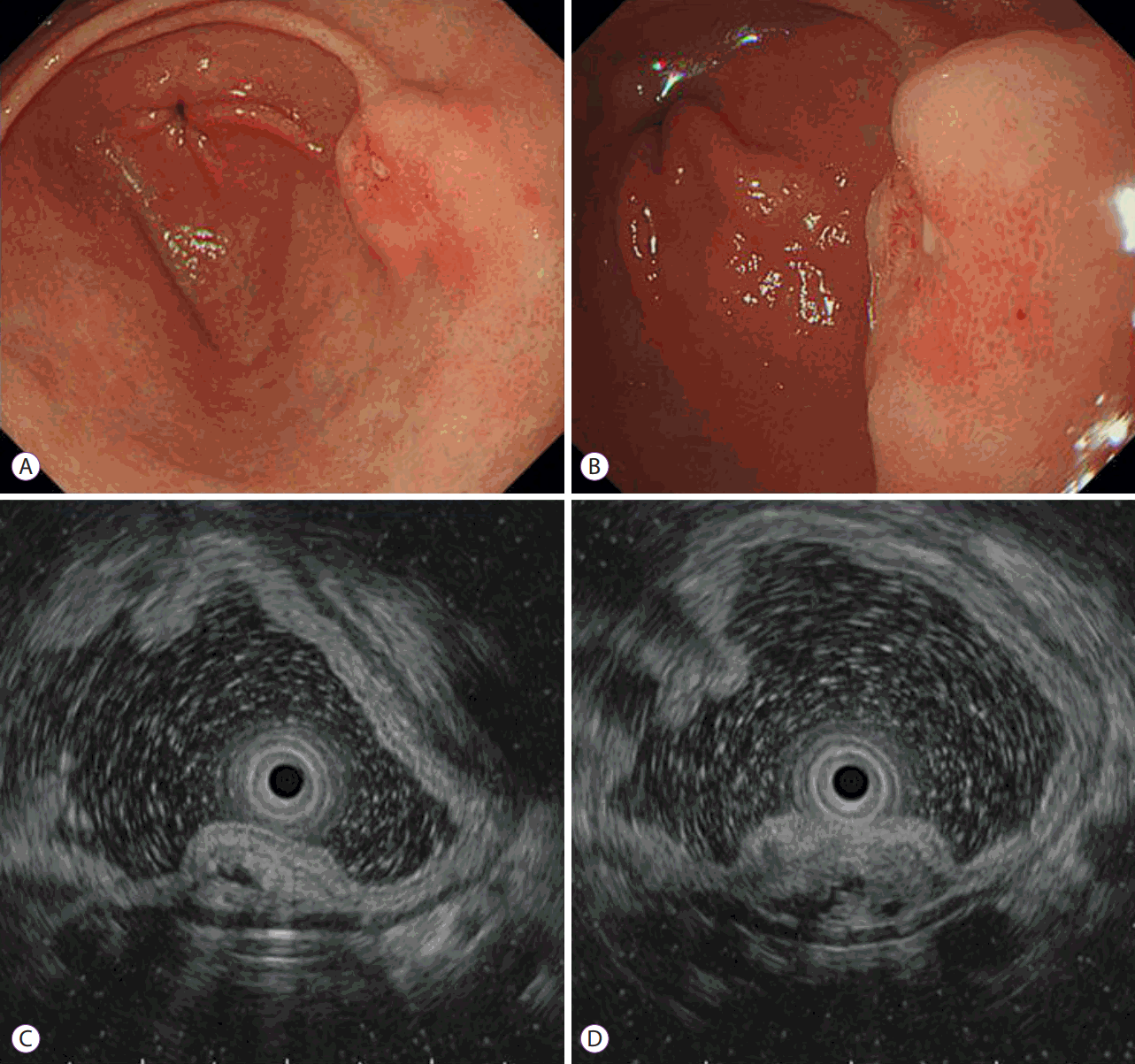 | Fig. 1.Endoscopic findings. (A, B) A subepithelial mass measuring 2.0×1.5 cm on the posterior wall of the distal antrum, and a 1-cm erythematous discoloration with erosion was noted on the surface of the mass. (C, D) Endoscopic ultrasonography revealed a 1.3×0.6-cm heterogeneously hypoechoic mass with indistinct margins located in the second and third layers of the gastric wall; anechoic cystic or tubular structures were observed within the mass, which were suggestive of heterotopic pancreas. The carcinoma appeared to be confined to the gastric mucosa. 
|
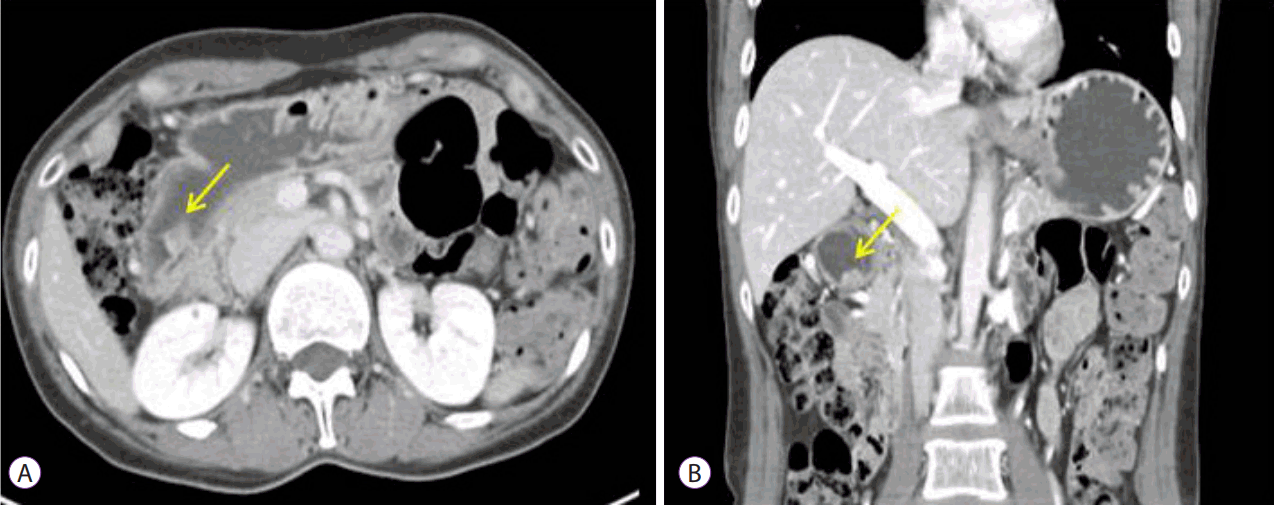 | Fig. 2.Abdominal computed tomographic findings. (A, B) A 1.4×1.0-cm protruding mass was observed at the distal antrum without lymph node or distant metastases. 
|
Distal gastrectomy was performed, which confirmed the presence of an early gastric cancer (0.5×0.3 cm) confined to the lamina propria without lymph node metastasis, and a separate underlying heterotopic pancreas (1.2×0.9 cm) (
Fig. 3A,
B). There was no evidence of dysplastic or malignant changes in the heterotopic pancreas (
Fig. 3C).
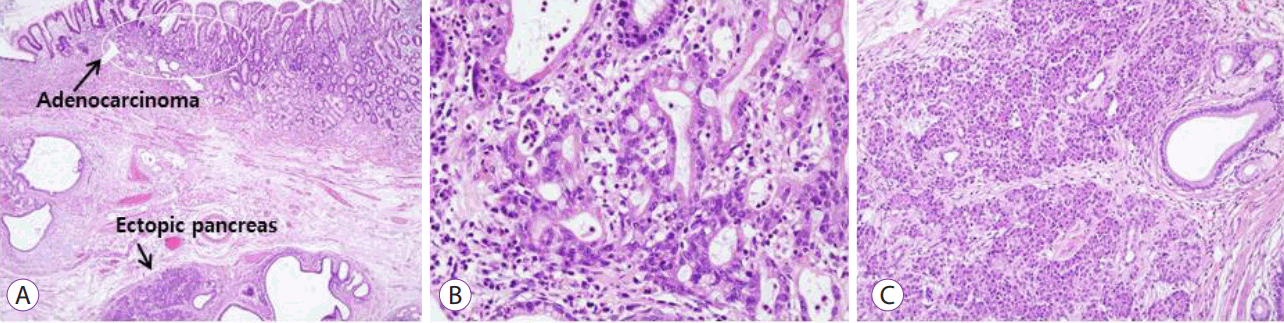 | Fig. 3.Microscopic findings. (A) An early gastric cancer (round mark) confined to the lamina propria and a separate underlying heterotopic pancreas were observed (Hematoxylin and eosin [H&E], ×40). (B) Atypical glandular cells showed hyperchromatic nuclei with prominent nucleoli, a finding that was consistent with moderately differentiated tubular adenocarcinoma (H&E, ×200). (C) The heterotopic pancreas was composed of acinar cells and ductal components, without dysplastic or malignant changes (H&E, ×200). 
|
Go to :

DISCUSSION
Heterotopic pancreas is a congenital anomaly caused by the detachment of pancreatic fragments from the main body of the organ, and their deposition at ectopic sites. It is thought to occur during foregut rotation, and the fusion of the dorsal and ventral pancreatic buds [
2]. Histologically, there are four types of heterotopic pancreata: type I (complete heterotopia), composed of all pancreatic cell types (acini, ducts, and islet cells); type II (canalicular heterotopia), composed of ductal components only; type III (exocrine heterotopia), composed of acinar cells only; and type IV (endocrine heterotopia), composed of islet cells only [
3]. Although heterotopic pancreas can occur anywhere along the gastrointestinal tract, the most common locations are the stomach (27.5%), duodenum (25.5%), and jejunum (15.9%). The incidence of heterotopic pancreas in the stomach reportedly ranges from 0.6% to 13.7% [
4]. Heterotopic pancreas in the stomach appears as a submucosal bulge covered with normal mucosa, sometimes with a central umbilication, and it frequently arises in the antrum along the greater curvature or posterior wall of the stomach. The umbilication, which may be the site of ductal drainage to the mucosal surface, is observed in less than half of the cases; therefore, it is important to differentiate it from other types of subepithelial lesions (e.g., gastrointestinal stromal tumors or metastatic carcinomas) [
5]. The characteristic features of endoscopic ultrasonography of heterotopic pancreas include heterogeneous echogenicity, indistinct borders, presence of an anechoic area, and location within the second, third, and/or fourth layers of the gastric wall [
6].
Heterotopic pancreas functions like a normal pancreas, and, hence, is susceptible to the diseases that affect the pancreas, such as pancreatitis and pseudocyst formation. Complications due to a mass effect may occur, including ulceration, bleeding, obstruction, and intussusception. However, the majority of cases of heterotopic pancreas are incidental findings without any clinical relevance.
Malignant transformation of heterotopic pancreas has rarely been documented in the literature, with the reported incidence ranging from 0.7% to 1.8% [
7,
8]. For diagnosis of a carcinoma arising from a heterotopic pancreas, the following three criteria must be met: (1) the carcinoma must be within or close to the heterotopic pancreatic tissue; (2) a direct transition must be observed between the pancreatic structures and the carcinoma; and (3) the non-neoplastic pancreatic tissue must comprise at least fully developed acini and ductal structures [
7]. Our present case clearly deviates from these criteria, because the early gastric cancer was pathologically independent of the underlying heterotopic pancreas. Moreover, there were no dysplastic or malignant changes in the heterotopic pancreas. Although a carcinoma arising from heterotopic pancreas is rare, an early gastric cancer overlying a heterotopic pancreas is even rarer. Murabayashi et al. first reported the occurrence of an early gastric cancer just above a heterotopic pancreas [
9]. In that case, the main endoscopic finding was early gastric cancer showing a 3-cm irregular ulcer; there was no distinct subepithelial lesion suggesting heterotopic pancreas, the presence of which was determined after surgery (a 1.2-cm heterotopic pancreas was confirmed). In our case, heterotopic pancreas was a major finding, along with a minute surface abnormality suggestive of early gastric cancer.
At present, the relationship between gastric cancer and heterotopic pancreas is unclear.
It is postulated that repeated inflammation or erosion caused by secreted pancreatic enzymes, or mucosa protrusion itself, promotes gastric carcinogenesis. Further research is necessary to establish a causal link between heterotopic pancreas and gastric cancer.
In conclusion, we report a unique case of an early gastric cancer overlying a heterotopic pancreas, which may have important clinical implications. Although heterotopic pancreas is usually a benign and uneventful condition, careful endoscopic observation of the mucosal surface is mandatory to avoid overlooking a coexisting early gastric cancer, and histologic examination should be performed if necessary.
Go to :

Notes
Go to :

ACKNOWLEDGMENTS
This work was supported by a Pusan National University Hospital Clinical Research Grant (2016).
Go to :

REFERENCES
1. Hickman DM, Frey CF, Carson JW. Adenocarcinoma arising in gastric heterotopic pancreas. West J Med. 1981; 135:57–62.
2. De Castro Barbosa JJ, Dockerty MB, Waugh JM. Pancreatic heterotopia; review of the literature and report of 41 authenticated surgical cases, of which 25 were clinically significant. Surg Gynecol Obstet. 1946; 82:527–542.
3. Hammock L, Jorda M. Gastric endocrine pancreatic heterotopia: report of a case with histologic and immunohistochemical findings and review of the literature. Arch Pathol Lab Med. 2002; 126:464–467.
4. Lack EE. Congenital and developmental abnormalities of the pancreas. In : Lack EE, editor. Pathology of the pancreas, gallbladder, extrahepatic biliary tract, and ampullary region. Oxford: Oxford University Press;2003. p. 44–62.
5. Song DE, Kwon Y, Kim KR, Oh ST, Kim JS. Adenocarcinoma arising in gastric heterotopic pancreas: a case report. J Korean Med Sci. 2004; 19:145–148.

6. Chou JW, Cheng KS, Ting CF, Feng CL, Lin YT, Huang WH. Endosonographic features of histologically proven gastric ectopic pancreas. Gastroenterol Res Pract. 2014; 2014:160601.

7. Guillou L, Nordback P, Gerber C, Schneider RP. Ductal adenocarcinoma arising in a heterotopic pancreas situated in a hiatal hernia. Arch Pathol Lab Med. 1994; 118:568–571.
8. Makhlouf HR, Almeida JL, Sobin LH. Carcinoma in jejunal pancreatic heterotopia. Arch Pathol Lab Med. 1999; 123:707–711.

9. Murabayashi T, Kawaguchi S, Okuda N, Oyamada J, Yabana T. Early gastric cancer just above a heterotopic pancreas. Case Rep Gastroenterol. 2016; 10:308–314.

Go to :







 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download