INTRODUCTION
Gastric subepithelial tumors (SETs) are identified as masslike lesions covered with normal mucosa from the gastrointestinal (GI) tract [
1,
2]. The prevalence of SETs in the GI tract is not well known. However, the detection rate of suspected gastric SETs with esophagogastroduodenoscopy (EGD) has previously been reported to be approximately 0.4% [
3]. In general, SETs are incidentally detected during endoscopy or identified using abdominopelvic computed tomography (APCT) without the presentation of specific symptoms [
4]. Because of recent advances in endoscopy and improvement in the quality of CT imaging, the detection of gastric SETs is expected to considerably increase. Gastric SETs include various types of tumors, ranging from benign to malignant [
1,
5]. Potentially malignant or malignant gastric SETs include gastrointestinal stromal tumors (GISTs), lymphomas, carcinoid tumors, and glomus tumors [
1]. These SETs should be differentiated for accurate diagnosis and appropriate treatment.
Currently, endoscopic ultrasonography (EUS) is the most sensitive imaging modality for the evaluation of gastric SETs [
6]. Owing to its high spatial resolution, EUS is able to provide information about the layer of origin, size, internal echogenicity, and echotexture of a tumor [
6,
7]. On the other hand, APCT, which is a less invasive modality, is widely conducted for abdominal assessments in clinical practice [
8]. Recent studies have reported that the accuracy of APCT in detecting gastric disease has increased with the improvement in CT imaging quality [
4,
8,
9]. APCT has unique advantages in the characterization of SETs based on CT attenuation values and evaluation of lymph node status [
10-
12]; however, it is unable to determine the layer of origin, which is an important factor in presumptive diagnosis [
9,
13]. Therefore, APCT is expected to play a more relevant role in the diagnosis of gastric SETs in the future. To date, the diagnostic ability of EUS and APCT with respect to gastric SETs has not been adequately compared, with only a few previous studies having reported the accuracy of APCT based on EUS findings as the standard of reference [
9,
13,
14]. This study therefore aimed to compare the diagnostic ability of EUS and APCT and to assess whether they are satisfactory modalities for diagnosing gastric SETs based on surgical histopathology results. In addition, cases of misdiagnosis with EUS were analyzed, and the causes of these misdiagnoses were identified.
Go to :

MATERIALS AND METHODS
Study subjects
We retrospectively investigated 53 patients who underwent laparoscopic wedge resection for suspected gastric SETs from January 2010 to December 2017 at Ewha Womans University Mokdong Hospital. All enrolled patients underwent both EGD and APCT for the evaluation of gastric SETs. On the basis of surgical histopathology results, we reviewed the presumptive diagnosis of preoperative EUS and APCT. The gastric SET cases were divided into 2 groups based on the histopathology results: benign and malignant/potentially malignant. Benign SETs mainly included leiomyomas, ectopic pancreas, schwannomas, lipomas, gastric duplication cysts (GDCs), and adenomyomas, whereas malignant/potentially malignant SETs included GISTs and lymphomas.
Endoscopic ultrasonography
EUS was performed by 2 investigators, each with >15 years of experience in gastroenterology. When a gastric SET was detected using EUS, the location (antrum, angle, body, cardia, or fundus), size, echotexture (homogeneous or heterogeneous), internal echogenicity (hypoechoic, hyperechoic, anechoic, or mixed), and layer of origin (mucosa, muscularis mucosa, submucosa, muscularis propria, or serosa) were described. Depending on the size and location, radial EUS (GF-UE260; Olympus Medical Systems Co., Tokyo, Japan) or a catheter probe (Olympus GIF 2T 240 with UM-3R) was used for 29 and 24 of the gastric SETs, respectively. During EUS, distilled water was injected through the endoscope, if needed.
Abdominopelvic computed tomography
APCT images were interpreted by 2 board-certified abdominal radiologists independently of the EUS results. They analyzed the size, location (antrum, angle, body, cardia, or fundus), enhancement degree (difference in Hounsfield units [HU] between the gastric wall and the lumen), enhancement pattern, and contour of the gastric SETs and provided an initial diagnosis. When the type of gastric SET was difficult to determine on APCT images owing to limited resolution capability, the tumor was classified as a possible gastric SET. APCT images were obtained using 64-section CT (Somatom Sensation 64; SIEMENS, München, Germany; n=42) and 128-section CT (Somatom Definition Flash; SIEMENS; n=11). The parameters for both 64-section and 128-section CT were as follows: slice thickness of 3 mm, peak voltage of 120 kVp, and injection of 120 mL of nonionic contrast material (Bonorex 350) at a rate of 2.0 mL/s. The tube currents of 64-section and 128-section CT were 170 and 150 mA, respectively. To ensure optimal conditions for gastric distension, all patients fasted for at least 8 h.
Assessment of diagnostic ability
We calculated the diagnostic ability (accuracy, sensitivity, specificity, positive predictive value [PPV], and negative predictive value [NPV]) of EUS and APCT for gastric SETs based on the surgical histopathology results, which were used to definitively diagnose the SETs.
Statistical analyses
The differences between benign gastric SETs and malignant/potentially malignant gastric SETs were analyzed using Student’s t-tests or Mann–Whitney U-tests and chi-squared tests. Student’s t-tests and chi-squared tests were used for continuous and categorical variables, respectively. Median (interquartile range) and nonparametric tests (Mann–Whitney U-tests) were used because the tumor size distribution was skewed. Statistical analysis was conducted using SPSS 21.0 for Windows (SPSS Inc., Chicago, IL, USA). A 2-tailed p-value of <0.05 was considered statistically significant.
Ethics statement
This study was approved by the Institutional Review Board of Ewha University Mokdong Hospital (approval no. 2018-08-052-001). Written informed consent was not obtained from the patients because this study had a retrospective design. The records of the patients were anonymized and de-identified before the analysis to protect their privacy. This study followed the Declaration of Helsinki.
Go to :

DISCUSSION
Because EUS can provide precise internal images of the layer of origin, it has been recognized as the most efficient diagnostic modality for gastric SETs [
1,
6]. However, APCT has other advantages in evaluating the characteristics, local extension, invasion to adjacent organs, or possible metastasis of SETs [
9]. Because of the different benefits of the 2 modalities, we evaluated the diagnostic ability of EUS and APCT for gastric SETs based on surgical histopathology results.
Consistent with previously reported results, we found that the overall accuracy of EUS in diagnosing gastric SETs was 64.2% [
15]. In comparison, APCT had an overall accuracy of 50.9%, which is somewhat lower than that of EUS. Both EUS and APCT demonstrated good results for the detection of GISTs in this study. Further, although both modalities exhibited low diagnostic accuracy for most benign gastric SETs, EUS was more reliable for leiomyomas and ectopic pancreas, whereas APCT was more acceptable for lipomas and GDCs.
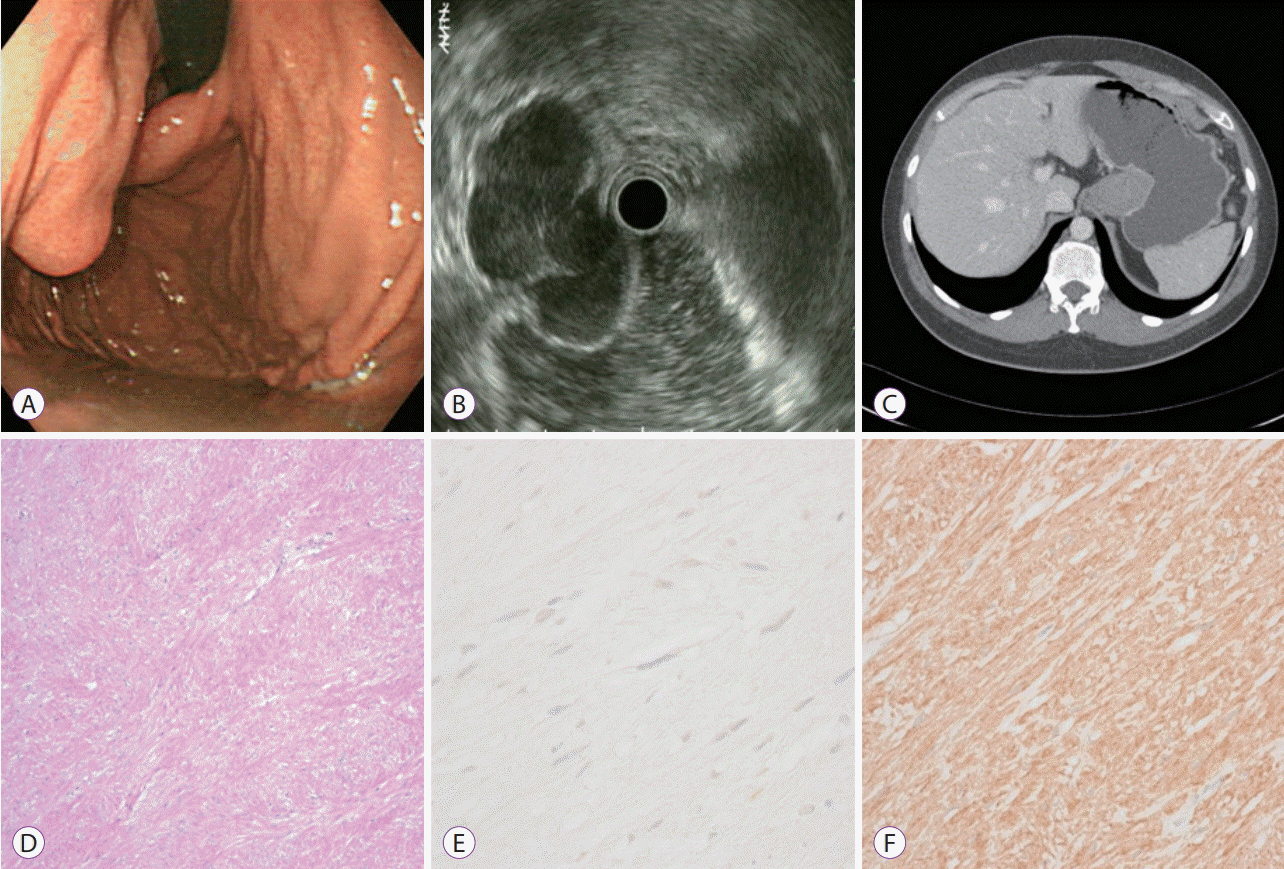
On EUS, GISTs are generally characterized as hypoechoic or having mixed echogenicity, having a homogeneous echotexture, and mainly originating in the fourth echolayer (corresponding to the muscularis propria) [
6,
16]. In addition, GISTs with high-risk features might present various EUS findings such as irregular shape, heterogeneous echotexture, echogenic foci, central necrosis, or calcification [
17]. In this study, 7 GISTs exhibited a homogeneous echotexture and 24 had a heterogeneous echotexture and various echogenicities including hyperechoic, hypoechoic, or mixed with echogenic foci or central necrosis. Most of these EUS findings closely corresponded with those of previous reports [
6,
16]. On APCT, GISTs usually present with a well-defined margin and homogeneous contrast enhancement [
14]. Previous studies have reported that attenuation values of 30–35 HU in pre-contrast imaging and 50–60 HU in post-contrast imaging are helpful in the differential diagnosis of GISTs using APCT [
10,
18]. APCT may also identify differences in appearance and enhancement based on the size of the GIST; in particular, for GISTs >5 cm, an ill-defined margin and heterogeneous enhancement with central necrosis or calcification were identifiable. In our study, 14 GISTs had a smooth margin and homogeneous contrast enhancement and 9 had an ill-defined margin and heterogeneous enhancement. It was also found that 8 GISTs had low or indefinite enhancement.
Hypoechoic echogenicity and diffuse thickening with fusion of the echolayers are usually reported as the key EUS features for diagnosing lymphoma [
19,
20]. However, as these are not always apparent in EUS, an accurate diagnosis is difficult with EUS alone. In the present study, EUS revealed a thickening of the third layer with multiple anechoic areas. Consequently, we identified lymphoma as gastritis cystica profunda. On APCT, lymphoma exhibits various imaging features, including single or multiple masses with or without ulcers, thickened folds, and mucosal nodularity of varying sizes [
21], which are characteristics similar to those exhibited by gastric carcinoma. A previous study demonstrated that the following features can strongly suggest a lymphoma: a bulky mass or diffuse infiltration with preservation of the fat planes, involvement of multiple sites, and lymphadenopathy [
22]. In our study, gastric wall thickening with preservation of the overlying mucosa and an accompanying lymphadenopathy were identified using APCT, which we considered possible indications of a lymphoma.
For benign gastric SETs, EUS is a more accurate and reliable diagnostic modality than APCT. Particularly for leiomyoma and ectopic pancreas, EUS may reveal subtle findings not seen with APCT. With EUS, leiomyomas can be characterized as well circumscribed, with a homogeneous echotexture and hypoechoic echogenicity and mainly originating in either the fourth or the second echolayer (corresponding to the muscularis mucosa) [
6]. In this study, the presumptive EUS diagnosis was correct for only 3 of the 8 leiomyoma cases because many of the EUS features associated with leiomyomas overlap with those of GISTs. Ectopic pancreas usually exhibits a heterogeneous echotexture and an anechoic ductal structure, while mostly developing in either the third (corresponding to the submucosa) or fourth echolayer [
23]. In our study, 3 cases of ectopic pancreas originated in the fourth echolayer, 3 cases in the third echolayer, and 1 case in the second echolayer. Moreover, 3 cases of ectopic pancreas were characterized by a mixed echogenicity and an anechoic ductal structure, whereas 4 exhibited a hypoechoic echogenicity. However, the accuracy of APCT was not satisfactory for either leiomyoma or ectopic pancreas. In the present study, APCT did not detect leiomyoma in 12.5% (1/8) and ectopic pancreas in 28.6% (2/7) of the patients because small lesions were obscured. Owing to the limited resolution of CT images, the 2 radiologists did not identify leiomyoma in 12.5% (1/8) and ectopic pancreas in 14.3% (1/7) of the patients. Although APCT features for benign gastric SETs are generally nonspecific, several studies have reported that leiomyoma and ectopic pancreas exhibit some characteristic CT features [
11,
12,
24,
25]. In a study comparing CT findings with pathologically proven gastric true leiomyoma, leiomyoma is homogeneous, has poor-to-moderate enhancement, and has low attenuation or iso-attenuation compared with muscle attenuation [
25]. However, we found that APCT did not provide valuable features for identifying leiomyomas to the same extent as it did for GISTs. In the diagnosis of ectopic pancreas, a previous study demonstrated that an antrum location, ill-defined margin, prominent enhancement of the overlying mucosa, and long diameter/short diameter ratio of >1.4 are specific APCT criteria [
11]. However, in the present study, 3 of the 7 ectopic pancreas cases were located in the body and 6 cases did not show a specific enhancement pattern.
On the other hand, the accuracy of APCT was acceptable for lipoma and GDC in this study. Lipoma typically presents with a well-circumscribed submucosal mass with uniform fat attenuation (70–120 HU), allowing a conclusive diagnosis to be made using APCT [
23,
26]. In addition, GDC can be diagnosed using APCT based on a well-defined, homogeneous, thickwalled cystic lesion with an enhanced inner lining, occasionally accompanied by calcification. These features have also been shown to be of diagnostic value for GDC in another study [
27]. Although APCT did not demonstrate satisfactory accuracy for benign gastric SETs, it can play a complementary role in providing additional characteristics for lipomas and GDCs.
We classified cases in which there was a discrepancy between the histopathology results and preoperative EUS diagnoses based on size, location, layer of origin, internal echogenicity, and echotexture. Most of these discrepancies involved hypoechoic lesions originating in the fourth echolayer in the body or cardia. These characteristics are commonly detected in mesenchymal tumors, including GISTs, leiomyomas, and schwannomas; thus, accurate diagnosis may be not easy in these cases. When gastric SETs are located in the cardia, access of the EUS probe is difficult. Consequently, the origin layer and internal echogenicity may not be clear and distinct in the cardia. In the present study, GISTs were commonly misdiagnosed as leiomyomas, and leiomyomas were commonly misdiagnosed as GISTs with EUS. Even when the size was >5 cm, a leiomyoma located in the cardia was often mistakenly assumed to be a GIST. Predicting the possibility of malignancy and making a clinical decision of whether to observe the progress of the lesion, to perform EUS-guided biopsy, or to choose resection are important [
16,
28]. In particular, laparoscopic wedge resection cannot be easily applied to gastric SETs located in the cardia because of the technical complexities of the procedure [
29]. These cases require invasive surgery and have a high risk of gastro-esophageal reflux as a complication. To avoid unnecessary surgery, EUS-guided biopsy should be considered when gastric SETs in the cardia exhibit malignant EUS features.
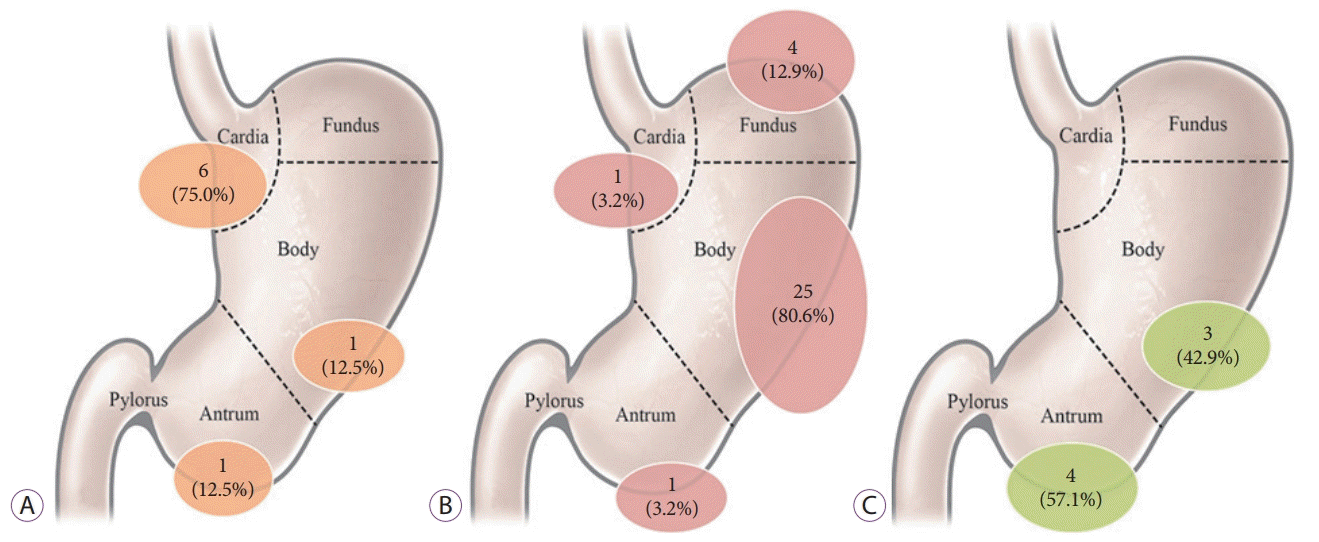
Previous studies have reported that the location, layer of origin, and shape of SETs can be used to predict their type [
30]. Our data revealed specific patterns for the location of gastric SETs that were similar to those reported in previous studies [
30-
32]. If a lesion is located in the cardia, the chance that it is a leiomyoma is high. GISTs are commonly located in the body and fundus, whereas ectopic pancreas is mainly located in the antrum. Although it cannot be applied to all types of gastric SETs, tumor location could be used to narrow down the differential diagnosis and could even be diagnostic in some cases.
Our study has some limitations. First, the potential for selection bias was present, as we performed a retrospective analysis and included only surgically resected cases. Second, the study was conducted at a single center; hence, interobserver variation could not be assessed. Third, the number of cases was relatively small. Therefore, a prospective study that includes a larger sample from multiple institutions is required to confirm our results. Finally, the 2 radiologists were blinded to the patients’ clinical information such as EGD findings. If the patients’ clinical background had been provided to the radiologists, the diagnostic accuracy of APCT could have been improved.
In conclusion, we investigated the diagnostic ability of EUS and APCT for gastric SETs relative to the conclusive diagnosis based on the surgical histopathology results. APCT exhibited lower overall accuracy than EUS. Nevertheless, APCT is a meritorious modality for malignant/potentially malignant gastric SETs including GISTs and lymphomas. APCT could also have a complementary role in providing valuable information on some benign gastric SETs such as lipomas and GDCs. For hypoechoic lesions originating in the fourth echolayer, EUS imaging alone is insufficient to accurately diagnose gastric SETs, although the location of gastric SETs could help in the differential diagnosis.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download