1. Wang J, Liu F, Gao H, et al. The symptom-to-treatment delay and stage at the time of treatment in cancer of esophagus. Jpn J Clin Oncol. 2008; 38:87–91.

2. Ishihara R, Takeuchi Y, Chatani R, et al. Prospective evaluation of narrow-band imaging endoscopy for screening of esophageal squamous mucosal high-grade neoplasia in experienced and less experienced endoscopists. Dis Esophagus. 2010; 23:480–486.
3. Muto M, Minashi K, Yano T, et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010; 28:1566–1572.

4. Yokoyama A, Ichimasa K, Ishiguro T, et al. Is it proper to use non-magnified narrow-band imaging for esophageal neoplasia screening? Japanese single-center, prospective study. Dig Endosc. 2012; 24:412–418.

5. Ebi M, Shimura T, Yamada T, et al. Multicenter, prospective trial of white-light imaging alone versus white-light imaging followed by magnifying endoscopy with narrow-band imaging for the real-time imaging and diagnosis of invasion depth in superficial esophageal squamous cell carcinoma. Gastrointest Endosc. 2015; 81:1355–1361. e2.

6. Tomie A, Dohi O, Yagi N, et al. Blue laser imaging-bright improves endoscopic recognition of superficial esophageal squamous cell carcinoma. Gastroenterol Res Pract. 2016; 2016:6140854.

7. Diao W, Huang X, Shen L, Zeng Z. Diagnostic ability of blue laser imaging combined with magnifying endoscopy for early esophageal cancer. Dig Liver Dis. 2018; 50:1035–1040.

8. Osawa H, Yamamoto H, Miura Y, et al. Blue laser imaging provides excellent endoscopic images of upper gastrointestinal lesions. Video Journal and Encyclopedia of GI Endoscopy. 2014; 1:607–610.

9. Ishihara R, Yamada T, Iishi H, et al. Quantitative analysis of the color change after iodine staining for diagnosing esophageal high-grade intraepithelial neoplasia and invasive cancer. Gastrointest Endosc. 2009; 69:213–218.

10. Goda K, Dobashi A, Yoshimura N, et al. Narrow-band imaging magnifying endoscopy versus Lugol chromoendoscopy with pink-color sign assessment in the diagnosis of superficial esophageal squamous neoplasms: a randomised noninferiority trial. Gastroenterol Res Pract. 2015; 2015:639462.

11. Oyama T, Inoue H, Arima M, et al. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: magnifying endoscopic classification of the Japan Esophageal Society. Esophagus. 2017; 14:105–112.

12. Kuehni RG. Color-tolerance data and the tentative CIE 1976 L a b formula. J Opt Soc Am. 1976; 66:497–500.
13. Shimizu Y, Omori T, Yokoyama A, et al. Endoscopic diagnosis of early squamous neoplasia of the esophagus with iodine staining: high-grade intra-epithelial neoplasia turns pink within a few minutes. J Gastroenterol Hepatol. 2008; 23:546–550.

14. Nagami Y, Tominaga K, Machida H, et al. Usefulness of non-magnifying narrow-band imaging in screening of early esophageal squamous cell carcinoma: a prospective comparative study using propensity score matching. Am J Gastroenterol. 2014; 109:845–854.

15. Maselli R, Inoue H, Ikeda H, et al. The metallic silver sign with narrow-band imaging: a new endoscopic predictor for pharyngeal and esophageal neoplasia. Gastrointest Endosc. 2013; 78:551–553.

16. Osawa H, Yamamoto H, Miura Y, et al. Diagnosis of depressed-type early gastric cancer using small-caliber endoscopy with flexible spectral imaging color enhancement. Dig Endosc. 2012; 24:231–236.

17. Fukuda H, Miura Y, Osawa H, et al. Linked color imaging can enhance recognition of early gastric cancer by high color contrast to surrounding gastric intestinal metaplasia. J Gastroenterol. 2018; Oct. 5. [Epub].
https://doi.org/10.1007/s00535-018-1515-6.

18. Osawa H, Miura Y, Takezawa T, et al. Linked color imaging and blue laser imaging for upper gastrointestinal screening. Clin Endosc. 2018; 51:513–526.

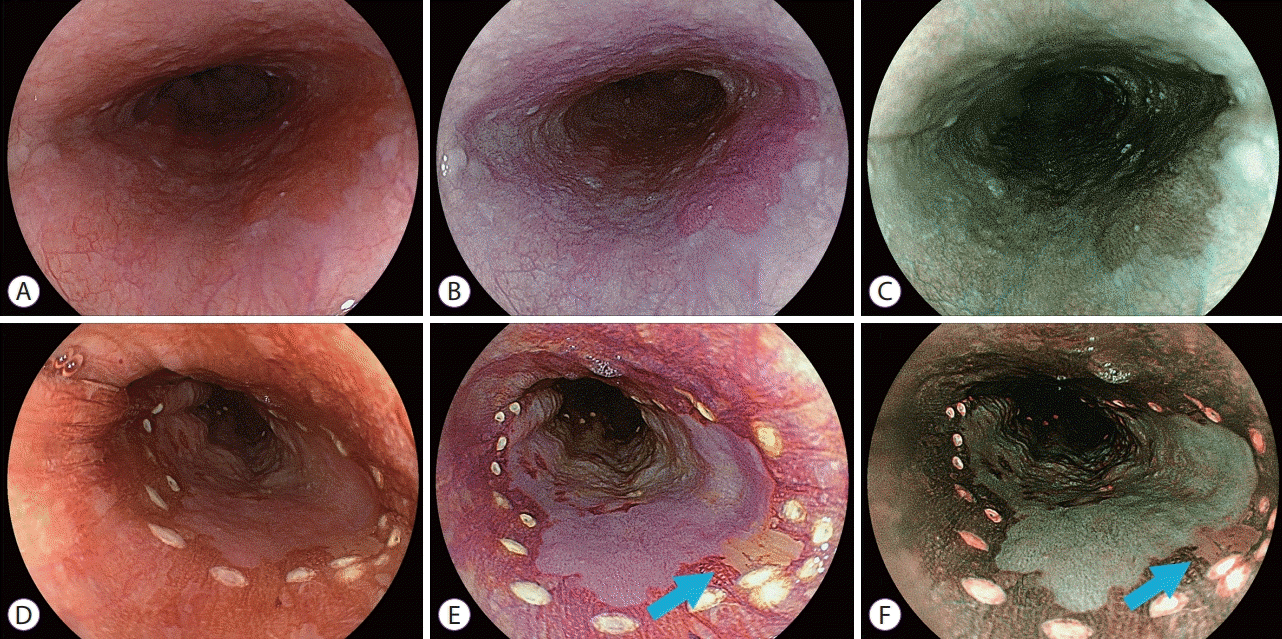
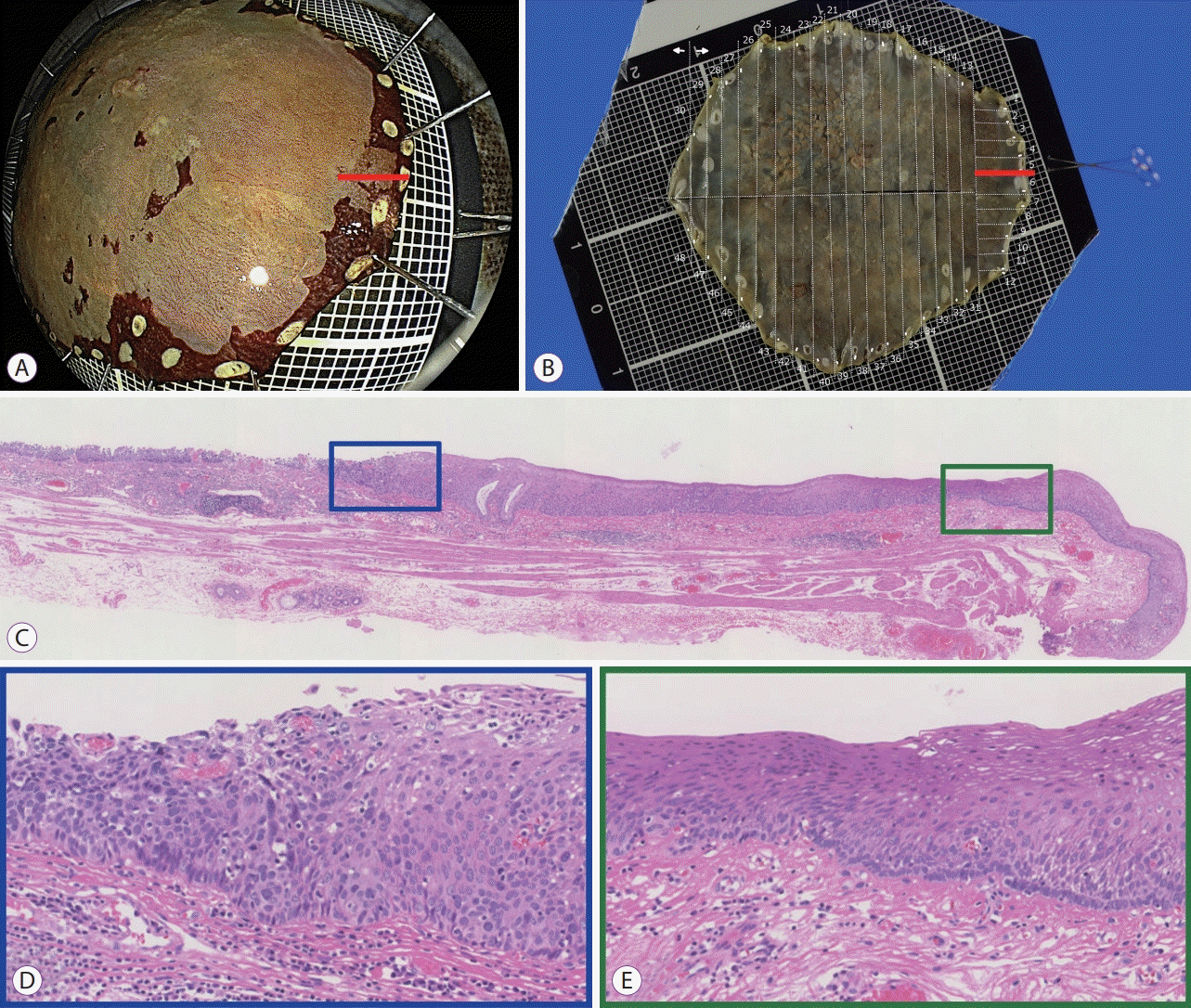




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download