INTRODUCTION
MATERIALS AND METHODS
Patients
Procedures of EUS
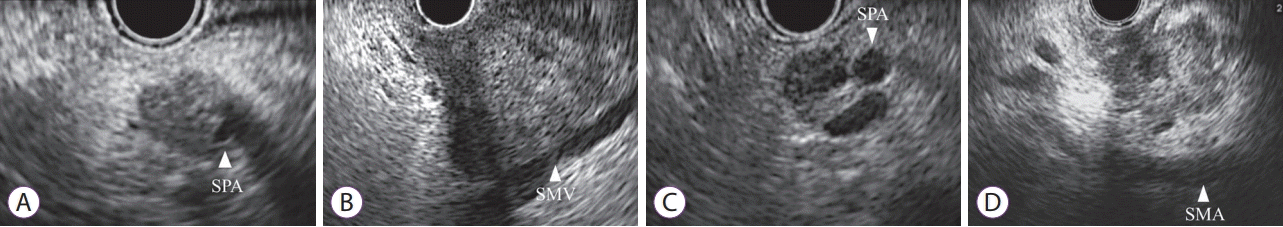 | Fig. 1.Classification of endoscopic ultrasonography findings into four types in accordance with the relationship between tumors and major vessels. (A) Type 1: clear invasion, encasement of vessel by tumor. (B) Type 2: a tumor that contacts a vessel with loss of hyperechoic vessel layer. (C) Type 3: a tumor that contacts a vessel without loss of hyperechoic vessel layer. (D) Type 4: clear non-invasion, existence of distance between a tumor and a vessel. SMA, superior mesenteric artery; SMV, superior mesenteric vein; SPA, splenic artery. |
Outcome measurements and definitions
Statistical analyses
RESULTS
Patient characteristics
Table 1.
Evaluation of vascular invasion by EUS
Table 2.
Table 3.
Table 4.
Relationship between EUS finding and pathologically evaluated distance between the tumor and vessel
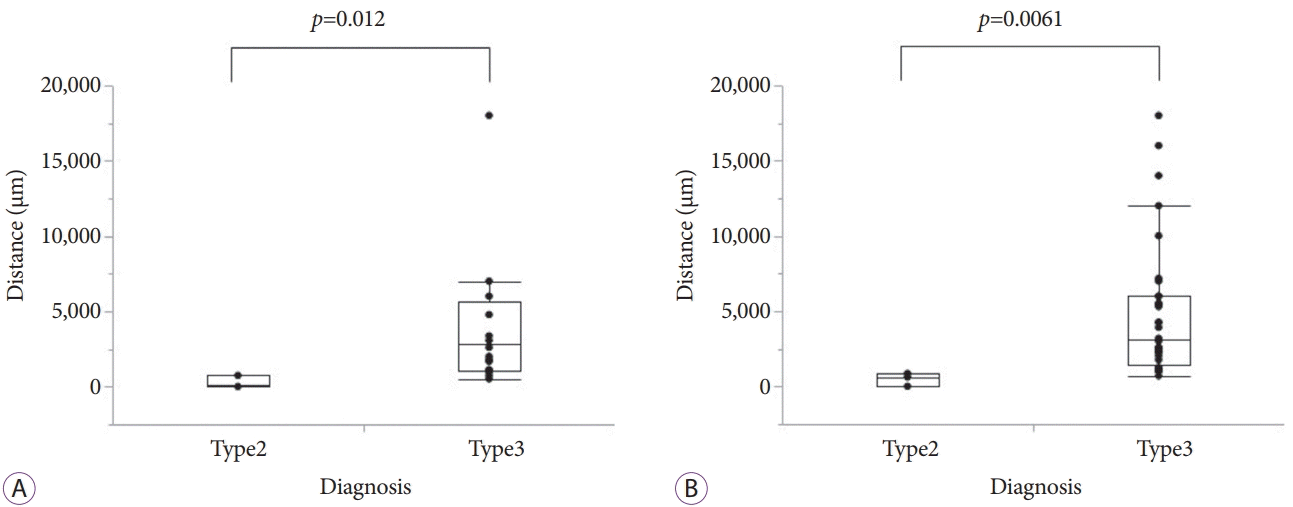 | Fig. 2.Box plots of distances between pancreatic cancers and pathologically evaluated vessels. Box plots of the distances from the evaluated major veins (A) and major arteries (B) to pancreatic cancers in cases of type 2 and type 3 endoscopic ultrasonography (EUS) findings with no vascular invasion. The distances of the cases with type 2 EUS finding were significantly shorter than those of cases with type 3 EUS finding in both, the veins and arteries. |
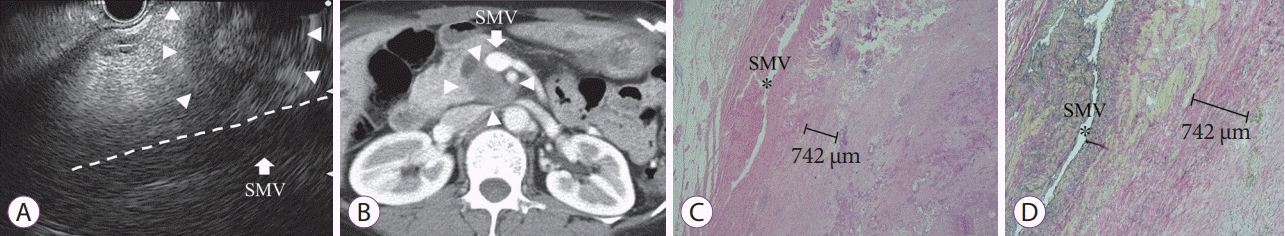 | Fig. 3.False-positive case of pancreatic cancer in transition of head and body, with a size of 55 mm. (A) This case was diagnosed as type 2. The pancreatic tumor (arrowhead) contacting the superior mesenteric vein (SMV: arrow) with the loss of the hyperechoic vessel layer, as observed by endoscopic ultrasonography. (B) Computed tomography image showing pancreatic tumor (arrowhead) contact SMV (arrow). (C) Pathological evaluation showing a distance of 742 µm between the tumor and SMV (hematoxylin-eosin stain, ×20). (D) No invasion to vessel was verified using Elastica van Gieson staining (×40). |
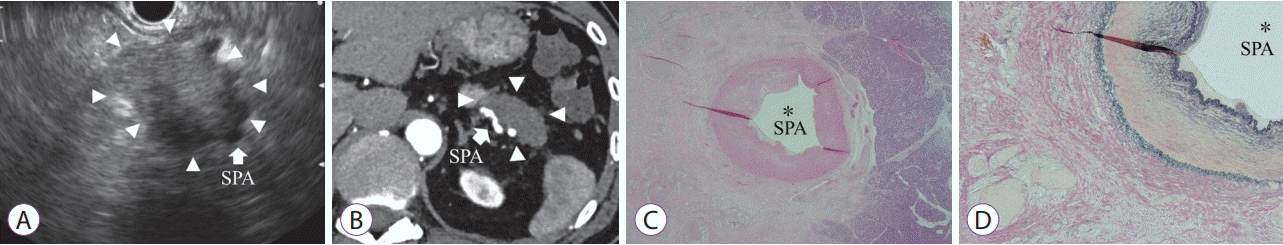 | Fig. 4.False-positive case of a pancreatic cancer in tail with a size of 45 mm. (A) This case was diagnosed as type 2. The pancreatic tumor (arrowhead) contacting the splenic artery (SPA: arrow), with the loss of the hyperechoic vessel layer, as observed by endoscopic ultrasonography. (B) Computed tomography image showing the pancreatic tumor (arrowhead) in contact with the SPA (arrow). (C) Pathological evaluation showing a distance of 0 μm between the tumor and SPA (hematoxylin-eosin staining, ×20). (D) Although proliferation of the tumor cells surrounding the SPA was observed, no invasion to vessel was verified in Elastica van Gieson staining (×40). |
Table 5.
| Patient No. | EUS finding | Evaluated vessel | Vascular invasion | Distance between tumor and vessel (μm) | Tumor location | Tumor size (mm) | Histological type |
|---|---|---|---|---|---|---|---|
| 1 | Type 2 | SMV | (–) | 742 | Ph (neck) | 55 | por |
| 2 | Type 2 | SMV | (–) | 96 | Ph (neck) | 33 | tub2 |
| 3 | Type 2 | SMV | (–) | 0a) | Pt | 65 | muc |
| 4 | Type 2 | SPA | (–) | 623 | Pb | 30 | tub2 |
| 5 | Type 2 | SPA | (–) | 0a) | Pt | 45 | tub2 |
| 6 | Type 2 | SMA | (–) | 854 | Ph (neck) | 25 | tub2 |
| 7 | Type 3 | PV | (+) | - | Ph | 26 | tub2 |
| 8 | Type 3 | PV | (+) | - | Ph | 45 | tub1 |
| 9 | Type 3 | SPA | (+) | - | Pt | 65 | muc |
Type 2: Loss of the extra-hyperechoic layer (positive invasion).
Type 3: Existence of the extra-hyperechoic layer of the vessel (negative invasion).
EUS, endoscopic ultrasonography; muc, mucinous adenocarcinoma; por, poorly differentiated adenocarcinoma; PV, portal vein; SMA, superior mesenteric artery; SMV, superior mesenteric vein; SPA, splenic artery; tub, tubular adenocarcinoma.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download