Abstract
Background/Aims
This study aimed to identify the predictive factors for inaccurate endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) diagnosis of swollen lymph nodes without rapid on-site cytopathological evaluation.
Methods
Eighty-three consecutive patients who underwent EUS-FNA for abdominal or mediastinal lymph nodes from January 2008 to June 2017 were included from a prospectively maintained EUS-FNA database and retrospectively reviewed. The sensitivity, specificity, and accuracy of EUS-FNA for the detection of neoplastic diseases were calculated. Candidate factors for inaccurate diagnosis (lymph node size and location, needle type, puncture route, number of passes, and causative disease) were evaluated by comparison between accurately diagnosed cases and others.
Results
The final diagnosis of the punctured lymph node was classified as neoplastic (65 cases: a metastatic lymph node, malignant lymphoma, or Crow-Fukase syndrome) or non-neoplastic (18 cases: a reactive node or amyloidosis). The sensitivity, specificity, and accuracy were 83%, 94%, and 86%, respectively. On multivariate analyses, small size of the lymph node was the sole predictive factor for inaccurate EUS-FNA diagnosis with a significant difference (odds ratios, 19.8; 95% confidence intervals, 3.15–124; p=0.0015).
Since endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for a pancreatic tumor was first reported in 1992 [1], it has been applied to many targets, such as subepithelial lesions of the gastrointestinal tract, mediastinal masses, biliary tumors, and ascites [2-12]. EUS-FNA can also be useful for diagnosing internal lymphadenopathy, which often requires correct diagnosis to confirm the cancer stage, recurrence of surgically resected malignancy, and non-neoplastic lymph nodes. Although several studies have reported the usefulness of EUS-FNA [13-19], its sensitivity for lymphadenopathy seems relatively lower than that for other targets, such as pancreatic masses [20-22].
The diagnostic accuracy of EUS-FNA can vary due to a few factors, including the target size and location, needle type, puncture route, number of needle passes, and causative disease [23]. Although rapid on-site evaluation (ROSE) during EUS-FNA has been reported to be associated with improved diagnostic yield [24-26], a recent randomized study has shown conflicting results regarding the impact of ROSE [27]. Moreover, that type of evaluation may be difficult to implement in most institutions due to the lack of a cytopathologist who is always available to perform EUS-FNA. If predictive factors for inaccurate results using EUS-FNA without ROSE can be identified, outcomes may improve with some countermeasures without on-site evaluation. Therefore, we sought to investigate these predictive factors in this study.
All patients who underwent EUS-FNA for swollen lymph nodes, which were detected in cross-sectional imaging examinations, at Sendai City Medical Center between January 2008 and June 2017 were extracted from the prospectively maintained EUS-FNA database and retrospectively reviewed. A swollen lymph node was defined as a node with a long axis size of at least 8 mm. Lymph-node EUS-FNA was performed in cases of swollen nodes with unknown etiology, those for which a determination of cancer stage was necessary, those with the possibility of cancer recurrence after surgical treatment, and those where the original cancer was difficult to puncture. Patients without sufficient procedural records and those without definitive final diagnoses because of a lack of follow-up were excluded. There were no patients aged <18 years and no occurrences of untreated coagulopathy or pregnancy during the study period. All patients provided written informed consent to undergo EUS-FNA. This study was approved by the institutional review board of our hospital.
EUS-FNA was performed under conscious or deep sedation with intravenous administration of midazolam, pentazocine, and/or propofol. Prophylactic antimicrobial agents were not administered. All patients were hospitalized to monitor possible adverse events for at least 1 night. After a swollen lymph node was visualized with a convex-arrayed echoendoscope (GF-UCT260, GF-UC240P; Olympus Co., Tokyo, Japan), an appropriate puncture route without intervening vessels was identified using the color Doppler mode. A 19-, 22-, or 25-G needle (EchoTip Ultra; Cook Japan Inc., Tokyo, Japan, EchoTip ProCore; Cook Japan Inc., Expect; Boston Scientific Japan K.K., Tokyo, Japan, EzShot 3 Plus; Olympus Co., SonoTip Pro Control; Medico’s Hirata Inc., Tokyo, Japan, or Acquire; Boston Scientific Japan K.K.) was inserted through the accessory channel of the scope and advanced into the target node. After the stylet was pulled out, 15 to 30 back-andforth movements of the needle were performed by suction with a 10- or 20-mL syringe. The needle was then withdrawn. The aspirated material was expelled into a receptacle with formalin solution using saline flushing. The puncture procedures were repeated until macroscopically visible whitish material was obtained but were limited to a maximum of 5 passes if no material was obtained. ROSE was not conducted for any patient. All procedures were carried out by an expert endosonographer (KI, SK, YK and TO) who had performed >500 EUS examinations and >50 EUS-FNA procedures or by a trainee supervised by experts. The needle type and size were determined at the endosonographer’s discretion. Adverse events related to EUS-FNA were defined as newly emerged complications that required prolongation of the hospitalization or therapeutic intervention. All EUS-FNA samples were prepared using the cell block method for microscopic evaluation [28]. Pathological evaluation was performed using hematoxylin- eosin staining, followed by additional immunochemistry and monoclonal antibody staining if desired.
The main outcome measurement of this study was the accuracy rate of EUS-FNA for swollen lymph nodes. Accuracy was defined as the precise diagnosis of neoplasm and non-neoplasm of the punctured node. Final diagnosis was classified into neoplastic and non-neoplastic lymph nodes, and the sensitivity and specificity for the detection of neoplastic lymph nodes were estimated. Neoplastic diseases included metastatic lymph nodes and hematopoietic/lymphoid neoplasms.
The final diagnosis was confirmed by pathological evaluation of a resected specimen when surgery was performed. For patients who did not undergo surgery, the diagnosis was defined as malignancy when pathological evaluation, such as EUS-FNA for the originating cancer and ascitic/pancreatic-juice cytology, confirmed the malignancy and the lymph node was larger on follow-up imaging evaluations. In patients without such pathological confirmation, the final diagnosis was defined as malignancy when the clinical course was sufficiently consistent, namely, when there were obvious findings in the imaging evaluation or when a patient’s condition continued to deteriorate, or they responded to antineoplastic treatment during the follow-up period of >12 months. The diagnosis was defined as non-neoplastic when the swollen lymph nodes regressed or disappeared during follow-up evaluation or were stable for >12 months.
The technical success of EUS-FNA was defined as the successful acquisition of macroscopically visible whitish material. The size and location of the punctured lymph node, needle type, needle diameter, puncture route, number of needle passes, and final diagnosis were evaluated to analyze the association between each factor and diagnostic accuracy using univariate and multivariate analyses.
The sensitivity, specificity, and accuracy rate of EUS-FNA for detecting neoplastic diseases were calculated with 95% confidence intervals (CI).
The above-mentioned candidate factors were compared between cases with accurate diagnosis and others. Analyses of predictive factors were performed using the Chi-squared test. Continuous variables were divided into 2 groups by using a cut-off value defined with the receiver operating characteristic (ROC) curve for multivariate analysis. Factors with a p-value <0.20 in the univariate analyses were evaluated in the multiple logistic regression model, and odds ratios (OR) with 95% CI were calculated. A two-tailed p-value <0.05 was considered statistically significant. The analyses were performed using JMP Pro13 for Mac (JMP 13; SAS Institute Inc., Cary, NC, USA).
After elimination of 6 patients without final diagnosis, 83 patients (male, 47; median age, 68 years [range, 22–88 years]) were included in this study. The final diagnosis was classified as neoplastic lymphadenopathy in 65 patients and non-neoplastic lymphadenopathy in 18 patients (Table 1). The origin of the metastatic lymph nodes was extrahepatic bile duct cancer in 17 patients, pancreatic cancer in 14, neuroendocrine tumor in 8, intrahepatic cholangiocarcinoma in 3, hepatocellular carcinoma in 2, gastric cancer in 2, lung cancer in 2, colon cancer in 1, duodenal cancer in 1, and an unknown cancer in 4. Details of the EUS-FNA procedures are shown in Table 2. Macroscopically visible material was obtained in all cases. No adverse events related to the procedure were observed.
The obtained materials were adequate for pathological evaluation in 93% (77/83) of the cases. Additional immunohistochemical staining was performed in 60/83 of the patients. Fifty-five of the specimens were pathologically determined to be neoplastic, while 22 were non-neoplastic. The EUS-FNA diagnosis accurately concurred with the final diagnosis in 71 cases. Of the 6 cases with inaccurate diagnosis, the EUS-FNA results were false-positive in 1 and false-negative in 5. In the single false-positive case, atypical lymphocytes obtained in EUS-FNA were positive for both CD20 and leukocyte common antigen, resulting in incorrect diagnosis as malignant lymphoma. However, the swollen lymph nodes, up to 15 mm in size, were obviously regressed to an undetectable level during follow-up without treatment, and the final diagnosis was judged as non-neoplastic reactive change. The 5 cases with false-negative EUS-FNA results can be described as follows. Two false-negative cases were diagnosed as having malignant lymphoma by bone marrow aspiration cytology. One case of pancreatic cancer was diagnosed by using EUS-FNA on the main tumor. One case of duodenal cancer was diagnosed by imaging examinations and clinical follow-up. Finally, 1 case of Crow-Fukase syndrome was diagnosed by evaluating surgically sampled lymph nodes. The EUS-FNA specimens of these false-negative cases contained several cell aggregations, which did not show malignant findings. In 6 other cases, EUS-FNA samples did not contain a sufficient number of neoplastic cells despite evaluating the macroscopically detectable materials on site. The final diagnoses were as follows: biliary tract cancer in 3 cases, pancreatic cancer in 2, and neuroendocrine tumor in 1.
In all the 83 cases evaluated, the overall sensitivity, specificity, and accuracy rate for the detection of a neoplastic lymph node were 83%, 94%, and 86%, respectively (Table 3).
On univariate analyses comparing accurately diagnosed cases and others, the size of the punctured lymph node was the sole predictive factor with a significant difference (median [interquartile range], 20 [16–25] mm vs. 14 [12–15] mm, p<0.0001) (Table 4).
In the ROC curve, the sensitivity and specificity were 76% and 83%, respectively, with a cut-off size of 16 mm (Fig. 1). The area under the receiver operator curve (AUC) was 0.76.
Multiple logistic regression showed that the lymph node size of <16 mm was the significant predictive factor for inaccuracy in EUS-FNA diagnoses (OR, 19.8; 95% CI, 3.15–124; p=0.0015) (Table 5).
Accurate diagnosis of neoplastic lymph nodes using EUSFNA appears to be relatively difficult in comparison with that of other neoplasms, such as pancreatic cancer, according to the results of previously reported studies [13-22] and the present study. If the predictive factors for unfavorable results of EUSFNA can be identified, diagnostic performance may improve. Hence, many previous studies have attempted to identify such factors [26,27,29-32].
Some studies have reported that on-site evaluation of the obtained samples by a cytopathologist improves the diagnostic yield of EUS-FNA for lymph nodes as well as pancreatic tumors [24-26,33-35], whereas Kappelle et al. recently reported that ROSE during EUS-FNA of lymph nodes did not improve the diagnostic yield in a multicenter, randomized trial [27]. In our study, the 6 cases (7.2%) without adequate specimens might have been successfully diagnosed via on-site evaluation. However, on-site evaluation is not available in most institutions due to limitations of human resources and costs.
The number of needle passes and the needle size are procedural factors that are associated with outcomes. When on-site evaluation is unavailable, the appropriate number of passes is always in question. Leblanc et al. [29] have reported that the number of needle passes (≥5) is the most significant influential factor on the diagnostic yields of EUS-FNA for lymph nodes without on-site cytopathologists. However, according to a randomized study by Wallace et al. [30], more than 3 passes are adequate. Pellisé et al. [31] have demonstrated that efficacy of needle pass repetition reaches a plateau at the third pass in lymph node EUS-FNA. Clearly, the optimal number of needle passes remains controversial. Although the reason why the number of needle passes was not a factor associated with accuracy in our study is unknown; for most of the patients (53/83), the number of needle passes was 3 or more, i.e., there might be a beta error.
On the contrary, in another study, a larger needle size was found to improve tissue acquisition in lymph node EUSFNA [13]. However, there have been no comparative studies in which the needle size was a significant predictive factor [26,32,36].
It seems particularly difficult to diagnose lymphoproliferative disorders with EUS-FNA specimens. Some studies have reported high diagnostic yields using flow cytometry for malignant lymphoma [13,14,37]. Although most lymphoproliferative diseases (malignant lymphoma and Crow-Fukase syndrome) were accurately diagnosed in our study, selective use of flow cytometry could improve diagnostic outcomes.
This is the first study in which the small size of the punctured lymph node was a predictive factor for inaccurate diagnoses. Metastatic cells in small lymph nodes, which are in an earlier period of metastasis, are thought to settle in a subcapsular sinus located in the marginal part of nodes [30,38-40]. However, it is extremely difficult to arbitrarily puncture a marginal part of such small nodes. Therefore, selecting larger nodes among multiple swollen ones may improve outcomes. In patients without lymph nodes larger than 16 mm, certain countermeasures, such as increasing needle passes, may be effective. Moreover, puncturing nodes with metastatic findings may improve the diagnostic outcomes. Metastatic lymph nodes have been reported to be round, larger than 10 mm, hypoechoic, and well-demarcated [41-44]. In addition, other studies have reported the usefulness of elastography and contrast enhancement in the harmonic mode to distinguish neoplastic from non-neoplastic nodes [45-47].
In addition, small lymph nodes can be reactively enlarged even if a neoplastic disease exists in another part of the body. The EUS-FNA results in this situation may have been false-negative in the present study due to our definition of a final diagnosis. Theoretically, the real accuracy could be revealed only in studies wherein the EUS-FNA results of the punctured lymph node are compared with the histological results of surgical sampling of the same node. However, such studies are extremely difficult to carry out because of ethical concerns. Performing surgery merely to sample lymph nodes should be avoided in patients with unresectable cancers due to lymph node metastasis or those with lymphoproliferative diseases that are treatable without lymph node sampling. Moreover, marking for confirmation of punctured lymph nodes is difficult and possibly invasive.
There are some limitations in this study. (1) This study was retrospectively conducted at a single center with a relatively small sample size. (2) Pathological confirmation with surgically resected specimens was not available in most cases because EUS-FNA revealed that the patients were inappropriate for surgery due to metastatic lymph nodes or lymphoproliferative diseases. Instead, sufficient clinical follow-up for >12 months was performed in all patients for final diagnosis. (3) Various types of FNA needles were used for EUS-FNA. The types and sizes of the needles were not included in the prospectively maintained database. Therefore, there was partial lack of needle data in this study; however, this should only minimally affect the results. Although the association between the needle type and outcomes cannot be clearly determined owing to the smaller sample size resulting from the elimination of cases with a lack of needle information in this study, this could be clarified in other studies wherein many different types of needles are not used. (4) The AUC value of 0.76 is relatively low. (5) Inadequate specimens led to 50% of the inaccurate diagnoses in this study.
In conclusion, the smaller size of the lymph node (<16 mm) was the only independent predictive factor associated with inaccurate EUS-FNA diagnosis of swollen lymph nodes without on-site evaluation. Future prospective studies with a large sample size are required to confirm these results.
REFERENCES
1. Wiersema MJ, Hawes RH, Tao LC, et al. Endoscopic ultrasonography as an adjunct to fine needle aspiration cytology of the upper and lower gastrointestinal tract. Gastrointest Endosc. 1992; 38:35–39.

2. Ardengh JC, Bammann RH, Giovani M, Venco F, Parada AA. Endoscopic ultrasound-guided biopsies for mediastinal lesions and lymph node diagnosis and staging. Clinics (Sao Paulo). 2011; 66:1579–1583.

3. Akahoshi K, Oya M, Koga T, et al. Clinical usefulness of endoscopic ultrasound-guided fine needle aspiration for gastric subepithelial lesions smaller than 2 cm. J Gastrointestin Liver Dis. 2014; 23:405–412.

4. Eckardt AJ, Jenssen C. Current endoscopic ultrasound-guided approach to incidental subepithelial lesions: optimal or optional? Ann Gastroenterol. 2015; 28:160–172.
5. Mekky MA, Yamao K, Sawaki A, et al. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010; 71:913–919.

6. Kaushik N, Khalid A, Brody D, McGrath K. EUS-guided paracentesis for the diagnosis of malignant ascites. Gastrointest Endosc. 2006; 64:908–913.

7. DeWitt J, Yu M, Al-Haddad MA, Sherman S, McHenry L, Leblanc JK. Survival in patients with pancreatic cancer after the diagnosis of malignant ascites or liver metastases by EUS-FNA. Gastrointest Endosc. 2010; 71:260–265.

8. DeWitt J, LeBlanc J, McHenry L, McGreevy K, Sherman S. Endoscopic ultrasound-guided fine-needle aspiration of ascites. Clin Gastroenterol Hepatol. 2007; 5:609–615.

9. Weilert F, Bhat YM, Binmoeller KF, et al. EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: results of a prospective, single-blind, comparative study. Gastrointest Endosc. 2014; 80:97–104.

10. Kim HJ, Lee SK, Jang JW, et al. Diagnostic role of endoscopic ultrasonography-guided fine needle aspiration of gallbladder lesions. Hepatogastroenterology. 2012; 59:1691–1695.

11. Hijioka S, Hara K, Mizuno N, et al. Diagnostic yield of endoscopic retrograde cholangiography and of EUS-guided fine needle aspiration sampling in gallbladder carcinomas. J Hepatobiliary Pancreat Sci. 2012; 19:650–655.

12. Ogura T, Kurisu Y, Masuda D, et al. Can endoscopic ultrasound-guided fine needle aspiration offer clinical benefit for thick-walled gallbladders? Dig Dis Sci. 2014; 59:1917–1924.

13. Yasuda I, Tsurumi H, Omar S, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy for lymphadenopathy of unknown origin. Endoscopy. 2006; 38:919–924.

14. Nakahara O, Yamao K, Bhatia V, et al. Usefulness of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for undiagnosed intra-abdominal lymphadenopathy. J Gastroenterol. 2009; 44:562–567.

15. Naini BV, Apple SK, Presley M, Moatamed NA. A correlation study on diagnostic endoscopic ultrasound-guided fine-needle aspiration of lymph nodes with histological and clinical diagnoses, the UCLA Medical Center experience. Diagn Cytopathol. 2008; 36:460–466.

16. Kurita A, Kodama Y, Nakamoto Y, et al. Impact of EUS-FNA for preoperative para-aortic lymph node staging in patients with pancreatobiliary cancer. Gastrointest Endosc. 2016; 84:467–475.e1.

17. Korenblit J, Anantharaman A, Loren DE, Kowalski TE, Siddiqui AA. The role of endoscopic ultrasound-guided fine needle aspiration (eusfna) for the diagnosis of intra-abdominal lymphadenopathy of unknown origin. J Interv Gastroenterol. 2012; 2:172–176.

18. Lee YN, Moon JH, Kim HK, et al. A triple approach for diagnostic assessment of endoscopic ultrasound-guided fine needle aspiration in pancreatic solid masses and lymph nodes. Dig Dis Sci. 2014; 59:2286–2293.

19. Iwashita T, Yasuda I, Doi S, et al. Endoscopic ultrasound-guided fine-needle aspiration in patients with lymphadenopathy suspected of recurrent malignancy after curative treatment. J Gastroenterol. 2009; 44:190–196.

20. Chen J, Yang R, Lu Y, Xia Y, Zhou H. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesion: a systematic review. J Cancer Res Clin Oncol. 2012; 138:1433–1441.

21. Puli SR, Bechtold ML, Buxbaum JL, Eloubeidi MA. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: a meta-analysis and systematic review. Pancreas. 2013; 42:20–26.
22. Banafea O, Mghanga FP, Zhao J, Zhao R, Zhu L. Endoscopic ultrasonography with fine-needle aspiration for histological diagnosis of solid pancreatic masses: a meta-analysis of diagnostic accuracy studies. BMC Gastroenterol. 2016; 16:108.

23. Savides TJ. Tricks for improving EUS-FNA accuracy and maximizing cellular yield. Gastrointest Endosc. 2009; 69(2 Suppl):S130–S133.

24. Matynia AP, Schmidt RL, Barraza G, Layfield LJ, Siddiqui AA, Adler DG. Impact of rapid on-site evaluation on the adequacy of endoscopic-ultrasound guided fine-needle aspiration of solid pancreatic lesions: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2014; 29:697–705.

25. Hébert-Magee S, Bae S, Varadarajulu S, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: a meta-analysis. Cytopathology. 2013; 24:159–171.

26. Cleveland P, Gill KR, Coe SG, et al. An evaluation of risk factors for inadequate cytology in EUS-guided FNA of pancreatic tumors and lymph nodes. Gastrointest Endosc. 2010; 71:1194–1199.

27. Kappelle WFW, Van Leerdam ME, Schwartz MP, et al. Rapid on-site evaluation during endoscopic ultrasound-guided fine-needle aspiration of lymph nodes does not increase diagnostic yield: a randomized, multicenter trial. Am J Gastroenterol. 2018; 113:677–685.

28. Noda Y, Fujita N, Kobayashi G, et al. Diagnostic efficacy of the cell block method in comparison with smear cytology of tissue samples obtained by endoscopic ultrasound-guided fine-needle aspiration. J Gastroenterol. 2010; 45:868–875.

29. LeBlanc JK, Ciaccia D, Al-Assi MT, et al. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004; 59:475–481.

30. Wallace MB, Kennedy T, Durkalski V, et al. Randomized controlled trial of EUS-guided fine needle aspiration techniques for the detection of malignant lymphadenopathy. Gastrointest Endosc. 2001; 54:441–447.

31. Pellisé Urquiza M, Fernández-Esparrach G, Solé M, et al. Endoscopic ultrasound-guided fine needle aspiration: predictive factors of accurate diagnosis and cost-minimization analysis of on-site pathologist. Gastroenterol Hepatol. 2007; 30:319–324.

32. Chin YK, Iglesias-Garcia J, de la Iglesia D, et al. Accuracy of endoscopic ultrasound-guided tissue acquisition in the evaluation of lymph nodes enlargement in the absence of on-site pathologist. World J Gastroenterol. 2017; 23:5755–5763.

33. Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of onsite cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003; 98:1289–1294.

34. Jhala NC, Jhala D, Eltoum I, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy: a powerful tool to obtain samples from small lesions. Cancer. 2004; 102:239–246.

35. Alsohaibani F, Girgis S, Sandha GS. Does onsite cytotechnology evaluation improve the accuracy of endoscopic ultrasound-guided fine-needle aspiration biopsy? Can J Gastroenterol. 2009; 23:26–30.

36. Jeong SH, Yoon HH, Kim EJ, Kim YJ, Kim YS, Cho JH. High-resolution endoscopic ultrasound imaging and the number of needle passages are significant factors predicting high yield of endoscopic ultrasound-guided fine needle aspiration for pancreatic solid masses without an on-site cytopathologist. Medicine (Baltimore). 2017; 96:e5782.

37. Ribeiro A, Vazquez-Sequeiros E, Wiersema LM, Wang KK, Clain JE, Wiersema MJ. EUS-guided fine-needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest Endosc. 2001; 53:485–491.

38. Faries MB, Bedrosian I, Reynolds C, Nguyen HQ, Alavi A, Czerniecki BJ. Active macromolecule uptake by lymph node antigen-presenting cells: a novel mechanism in determining sentinel lymph node status. Ann Surg Oncol. 2000; 7:98–105.

39. Carter BA, Jensen RA, Simpson JF, Page DL. Benign transport of breast epithelium into axillary lymph nodes after biopsy. Am J Clin Pathol. 2000; 113:259–265.

40. Hartveit F. Micrometastases to the axilla in breast cancer: their size and season of presentation. Invasion Metastasis. 1996; 16:144–149.
41. Wiersema MJ, Hassig WM, Hawes RH, Wonn MJ. Mediastinal lymph node detection with endosonography. Gastrointest Endosc. 1993; 39:788–793.

42. Catalano MF, Sivak MV Jr, Rice T, Gragg LA, Van Dam J. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc. 1994; 40:442–446.

43. Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997; 45:474–479.

44. Choudhary NS, Bodh V, Kumar N, et al. Yield of endoscopic ultrasound-guided fine needle aspiration for subcentimetric lymph nodes: a comparison to larger nodes. Endosc Ultrasound. 2017; 6:168–173.

45. Sazuka T, Akai T, Uesato M, et al. Assessment for diagnosis of lymph node metastasis in esophageal cancer using endoscopic ultrasound elastography. Esophagus. 2016; 13:254–263.

Fig. 1.
The receiver operating characteristic (ROC) curve showing the relationship between lymph node size and the diagnostic yields of endoscopic ultrasound-guided fine needle aspiration for lymph nodes. Area under the ROC curve=0.76.
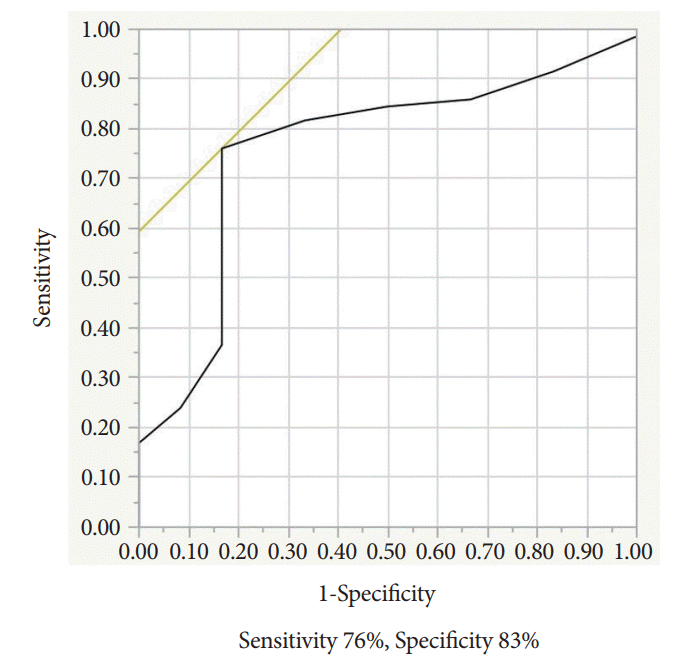
Table 1.
Characteristics of the Patients and Lesions
Table 2.
2. Details of Endoscopic Ultrasound-Guided Fine Needle Aspiration Procedures
Table 3.
Diagnostic Yields of Endoscopic Ultrasound-Guided Fine Needle Aspiration for Lymph Nodes
| 95% CI | ||
|---|---|---|
| Sensitivity | 83% | 78–84 |
| Specificity | 94% | 77–99 |
| Accuracy | 86% | 78–88 |
Table 4.
Univariate Analysis for Predictive Factors Associated with Diagnostic Accuracy of Endoscopic Ultrasound-Guided Fine Needle Aspiration
Table 5.
Logistic Regression Analysis for Predictive Factors Associated with Diagnostic Accuracy of Endoscopic Ultrasound-Guided Fine Needle Aspiration
| OR (95% CI) | p-value | |
|---|---|---|
| Lymph node diameter (≥16 mm) | 19.8 (3.15–124) | 0.0015 |
| Location of lymph node | 2.48 (0.49–12.6) | 0.27 |
| No. of needle passes | 2.54 (0.18–35.7) | 0.49 |
| Final diagnosis | 5.08 (0.48–53.3) | 0.17 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download