This article has been
cited by other articles in ScienceCentral.
Quiz
A laterally spreading tumor was found during a screening colonoscopy in a 71-year-old woman. The lesion was flat and irregular shaped, 20 mm × 30 mm in size, and located 10 cm above the anal verge. There was no ulcer or erosion on the lesion. For histologic diagnosis and therapeutic purpose, a piecemeal endoscopic mucosal resection was performed. The endoscopic and histologic images are shown in
Fig. 1.
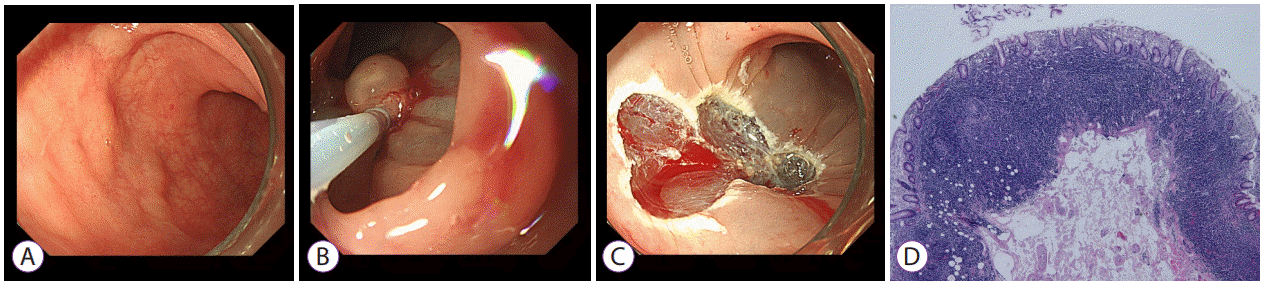 | Fig. 1.Colonoscopy and microscopy findings. (A-C) Colonoscopy revealed a flat, irregularly marginated, and superficially elevated lesion at the mid-rectum for which a piecemeal endoscopic mucosal resection was performed; (D) Microscopy revealed dense lymphoid infiltration involving the mucosa and submucosa (hematoxylin and eosin, ×40). 
|
What is the most likely diagnosis?
Go to :

Answer
The immunohistochemistry results are described in
Fig. 2.
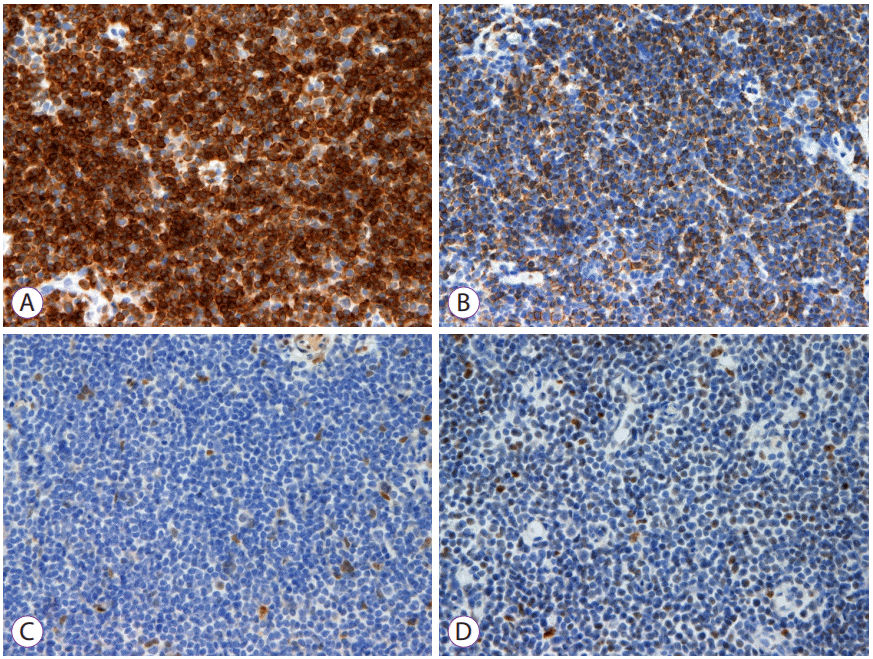 | Fig. 2.Immunohistochemistry results. (A) Positive for CD20; (B) Positive for Bcl-2; (C) Negative for cyclin D1; (D) Negative for Bcl-6. 
|
Histopathological examination of the specimen revealed that small-sized lymphocytes infiltrated into the mucosal and submucosal layers. The results of the immunohistochemical stain were as follows: CD5(–), CD20(+), cyclin D1(–), Bcl-2(+), and Bcl-6(–). An appropriate diagnosis is colonic mucosa-associated lymphoid tissue (MALT) lymphoma because tumor cells were positive for CD20 and Bcl-2, whereas negative for cyclin D1 and Bcl-6. For staging, abdominopelvic computed tomography, chest computed tomography, and positron emission tomography-computed tomography were performed. No metastatic lesions were found. The patient is currently undergoing follow-up in outpatient clinic without progression of the disease.
MALT lymphoma most often occurs in the stomach, in addition to other organ systems such as the thyroid and salivary glands, lungs, and breasts [
1]. Gastrointestinal MALT lymphomas can occur in the small and large intestines in addition to the stomach. About 80% of gastric MALT lymphomas are thought to be associated with
Helicobacter pylori infections. Chronic inflammation of epithelial cells due to a
H. pylori infection causes proliferation of T cells in the mucous membrane and proliferation of B cells. Among them, the proliferation of B cells with genetic mutations results in MALT lymphoma. In addition, MALT lymphomas have been reported to be related to
Borrelia burgdorferi,
Chlamydia jejuni, and
hepatitis C virus infections and some autoimmune diseases [
2].
Systemic symptoms, such as B symptoms, are rare because colonic MALT lymphomas have a slow-growing nature and remain in the local area. Colonic MALT lymphoma is most commonly found around the ileocecal valve area, often in order of occurrence starting from the rectum, ascending colon, transverse colon, sigmoid colon, and descending colon.
Endoscopic findings of colonic MALT lymphomas are predominantly in the form of a single polyp and sessile elevated mucosal lesion [
3]. The endoscopic findings of gastric MALT lymphomas can be diverse, such as superficial ulcers, irregular mucosa, atrophic mucosa, bumps, and erosion. Gastrointestinal MALT lymphoma is diagnosed by an endoscopic biopsy and immunohistochemical staining for suspected lesions. Gastrointestinal MALT lymphoma can be diagnosed by lymphoepithelial lesions due to infiltration of atypical lymphocytes, centrocyte-like cells, and plasma cell differentiation as well as by cells that are positive for CD19, CD20, CD22, and bcl-2 and negative for CD3, CD5, CD10, and bcl-1/cyclin D1 [
4]. The MALT lymphoma shows positive for B cell markers (CD19, CD20, and CD22), and low-grade B-cell non-Hodgkin lymphoma among the extranodal marginal zone lymphomas has similar immunohistochemical staining results of being negative for CD5, CD10, and CD23.
In addition, endoscopic ultrasound, computed tomography, and bone marrow biopsy are performed for staging.
Treatment of gastrointestinal MALT lymphoma depends on the stage. In cases of gastric MALT lymphoma, H. pylori eradication is performed first at an early stage, and radiotherapy and chemotherapy are administered in cases of initial treatment failure. The effectiveness of H. pylori eradication in MALT lymphomas occurring in areas other than the stomach varies. For Colorectal MALT lymphomas, there are yet no clear treatment guidelines and various treatments such as endoscopic resection, surgery, combined chemotherapy, local radiation therapy, and H. pylori eradication are used [
5,
6].
Go to :






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download