Abstract
The novel coronavirus disease (COVID-19) quickly spread to all continents. However, data regarding all the signs and symptoms of COVID-19 are insufficient. Patients with COVID-19 might present higher susceptibility to fungal coinfections. Mucormycosis is a rare and often life-threatening fungal disease characterized by vascular invasion by hyphae, resulting in thrombosis and necrosis. This is the first case report of mucormycosis in a COVID-19 patient. An 86-year-old male patient was admitted to the emergency room with acute diarrhea, cough, dyspnea, and fever from 5 days prior. Blood tests revealed a hemoglobin level of 14.3 mg/dL. Five days following the admission, the patient presented with melena and a hemoglobin level of 5.6 mg/dL. A transfusion of three units of red blood cells was required. Esophagogastroduodenoscopy revealed two giant gastric ulcers with necrotic debris and a deep hemorrhagic base without active bleeding. Furthermore, biopsies confirmed mucormycosis. Despite intensive care, the patient died 36 hours after the esophagogastroduodenoscopy.
Mucormycosis (previously called zygomycosis) is a rare and devastating disease caused by a ubiquitous fungus that belongs to the class Zygomycetes and order Mucorales [1]. The clinical presentation of mucormycosis varies depending on the location of the disease [2]. The majority of patients with invasive mucormycosis are either undergoing treatment with immunosuppressants or have underlying conditions, such as diabetes mellitus, hematologic malignancies, and trauma or are transplant recipients. Outbreaks of the fungal disease have also been associated with natural disasters [3-5].
Although unusual, mucormycosis of the gastrointestinal (GI) tract occurs as a result of the ingestion of the spores of the fungus. Cases of GI mucormycosis in an immunocompetent host are rarely reported; however, the mortality rate due to GI mucormycosis can be as high as 85% [6].
In early December 2019, the first cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection were identified in Wuhan [7,8]. As this is a relatively novel virus, data regarding all the signs indicative of coronavirus disease (COVID-19) and symptoms that can be caused by the disease are insufficient [9]. Furthermore, little is known about other diseases that can increase the risk of SARS-CoV-2 coinfection. To the best of our knowledge, this is the first case of mucormycosis reported in a COVID-19 patient.
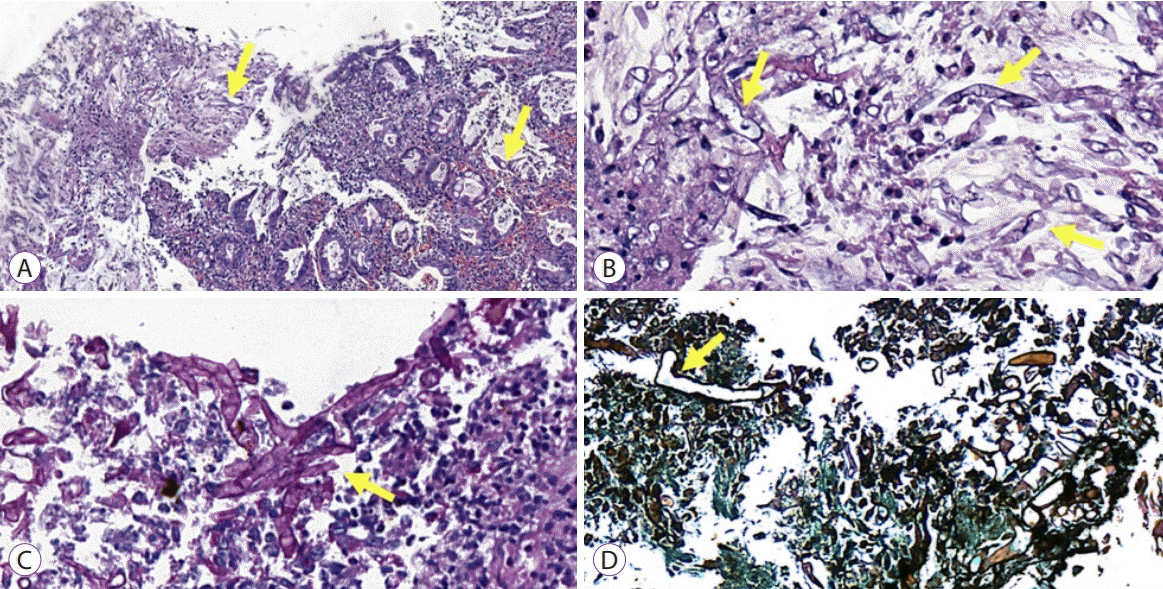
An 86-year-old male patient with a history of arterial hypertension was admitted to the emergency room with acute diarrhea, cough, dyspnea, and fever that started 5 days before admission. Blood analysis revealed a hemoglobin level of 14.3 mg/dL, a leukocyte count of 11.2×109/L, lymphocyte count of 0.58×109/L, a D-dimer level of 602 ng/mL, a lactate dehydrogenase level of 850 U/L, a creatinine level of 2.34 mg/dL, and a urea level of 93 mg/dL. A throat swab was collected from the patient, which confirmed COVID-19. Computed tomography of the chest was performed, and the findings are demonstrated in Fig. 1. Due to acute respiratory failure and hemodynamic instability, he was transferred to the intensive care unit (ICU). The patient was initially treated with ceftriaxone, azithromycin, oseltamivir, and hydrocortisone, besides intensive care management including vasopressors and mechanical ventilation. Five days following ICU admission, the patient presented with melena and severe anemia (hemoglobin level of 5.6 mg/dL). Physical examination revealed mild abdominal tenderness. He was managed with three units of red blood cells and omeprazole. Esophagogastroduodenoscopy (EGD) revealed two giant gastric ulcers with dirty debris and a deep hemorrhagic base without active bleeding located in the greater and lesser curvature (Fig. 2). Pathological examination confirmed mucormycosis (Fig. 3). Unfortunately, the patient died 1 week following hospitalization, 36 hours after the EGD, and before a diagnosis was established. Antifungal agents were not administered.
The ongoing outbreak of COVID-19 originated in Wuhan, China, in December 2019. COVID-19, which is the disease associated with SARS-CoV-2 infection, spread rapidly to produce a global pandemic [10]. The spectrum of symptomatic SARS-CoV-2 infection ranges from mild to critical. The proportion of severe or fatal infections may also vary by location.
Patients with COVID-19 might present with markedly higher levels of inflammatory cytokines (such as interleukin [IL]-2R, IL-6, IL-10, and tumor necrosis factor-alpha), associated with impaired cell-mediated immune response, affecting both CD4 + T and CD8 + T cells. Hence, an increased susceptibility to fungal coinfections is observed [11].
Mucormycosis is characterized by infarction and necrosis of the host tissues that result from an invasion of the vasculature by hyphae. Depending on the anatomic site involved, mucormycosis can present as a variety of different syndromes, including rhino-orbital-cerebral, pulmonary, cutaneous, and less often GI, renal, and disseminated diseases [12]. GI involvement presents in approximately 8% of the cases. The most commonly affected organs in the GI tract include the stomach and colon.
The symptoms of GI mucormycosis may include fever, nausea, abdominal pain, GI bleeding, and perforation. Establishing a correct diagnosis is challenging due to the nonspecific clinical signs/symptoms, which may not result in suspicions of mucormycosis. The endoscopic appearance of gastric mucormycosis is usually a large ulcer with necrosis, eventually presenting an adherent, thick, green exudate [13,14]. Diagnosis is confirmed by histopathologic identification based on the biopsy of the suspected area during surgery or endoscopy [6]. The disease has a high fatality rate (up to 85%) related to late or no diagnosis [15].
Treatment involves surgical debridement of involved tissues and antifungal therapy. Intravenous amphotericin B (a lipid formulation) is the drug of choice for initial therapy [16]. Despite early diagnosis and aggressive combined surgical and medical therapy, the prognosis for recovery from mucormycosis is poor [6].
We presented a case of an elderly patient whose only known comorbidity was arterial hypertension, without traditional risk factors for developing mucormycosis. He was admitted following the diagnosis of COVID-19 and acute respiratory failure and managed with broad-spectrum antibiotics and corticosteroids (which are risk factors for invasive fungal disease) [17]. It is known that COVID-19 may be associated with immune dysregulation [18], and it has been reported that patients with COVID-19 might be at risk of developing invasive fungal infections, such as invasive aspergillosis, candidiasis, and Pneumocystis jiroveci infection [11,19]. However, there are no reports of mucormycosis associated with COVID-19. Our patient was diagnosed with primary gastric mucormycosis, but it is unknown whether the cause of death was invasive mucormycosis.
GI mucormycosis is a rare disease, and it should be considered if an atypical gastric ulcer is identified in a COVID-19 patient. Clinical suspicion and prompt treatment are fundamental to achieve the cure of the disease. Hence, preemptive therapy should be considered in the presence of features suggesting mucormycosis. More studies are necessary to determine if these two pathologies are related.
Notes
Conflicts of Interest: Eduardo Guimarães Hourneaux de Moura reports personal fees from Boston Scientific and Olympus outside the submitted work. The other authors have no financial conflicts of interest.
Author Contributions
Conceptualization: Epifanio Silvino do Monte Junior, Igor Braga Ribeiro
Data curation: ESMJ
Formal analysis: Marcos Eduardo Lera dos Santos, IBR, Gustavo de Oliveira Luz, Elisa Ryoka Baba
Investigation: ESMJ, MELS, Bruno Salomão Hirsch, Mateus Pereira Funari
Supervision: MELS, IBR, GOL, ERB, Eduardo Guimarães Hourneaux de Moura
Writing-original draft: ESMJ
Writing-review&editing: IBR
REFERENCES
1. Adhikari S, Gautam AR, Paudyal B, Sigdel KR, Basnyat B. Case report: gastric mucormycosis- a rare but important differential diagnosis of upper gastrointestinal bleeding in an area of Helicobacter pylori endemicity. Wellcome Open Res. 2019; 4:5.

2. Kauffman CA, Malani AN. Zygomycosis: an emerging fungal infection with new options for management. Curr Infect Dis Rep. 2007; 9:435–440.

4. Jeong W, Keighley C, Wolfe R, et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019; 25:26–34.

5. Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the mycoses study group education and research consortium. Lancet Infect Dis. 2019; 19:e405–e421.
6. Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005; 41:634–653.

7. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382:1708–1720.

8. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020; 109:102433.

9. Moura DTH, McCarty TR, Ribeiro IB, et al. Diagnostic characteristics of serological-based COVID-19 testing: a systematic review and meta-analysis. Clinics (Sao Paulo). 2020; 75:e2212.

10. Broughton JP, Deng X, Yu G, et al. CRISPR-Cas12-based detection of SARS-CoV-2. Nat Biotechnol. 2020; 38:870–874.

11. Song G, Liang G, Liu W. Fungal co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia. 2020; 185:599–606.

12. Riley TT, Muzny CA, Swiatlo E, Legendre DP. Breaking the mold: a review of mucormycosis and current pharmacological treatment options. Ann Pharmacother. 2016; 50:747–757.
13. Cherney CL, Chutuape A, Fikrig MK. Fatal invasive gastric mucormycosis occurring with emphysematous gastritis: case report and literature review. Am J Gastroenterol. 1999; 94:252–256.

14. de Moura DTH, Proença IM, McCarty TR, et al. Gastrointestinal manifestations and associated health outcomes of COVID-19: a Brazilian experience from the largest South American public hospital. Clinics (Sao Paulo). 2020; 75:e2271.

15. Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012; 54(Suppl 1):S23–S34.

16. Spellberg B, Walsh TJ, Kontoyiannis DP, Edwards J Jr, Ibrahim AS. Recent advances in the management of mucormycosis: from bench to bedside. Clin Infect Dis. 2009; 48:1743–1751.

17. Salehi M, Ahmadikia K, Badali H, Khodavaisy S. Opportunistic fungal infections in the epidemic area of COVID-19: a clinical and diagnostic perspective from Iran. Mycopathologia. 2020; 185:607–611.

Fig. 1.
The chest computed tomography revealed ground-glass opacity with consolidative abnormalities.
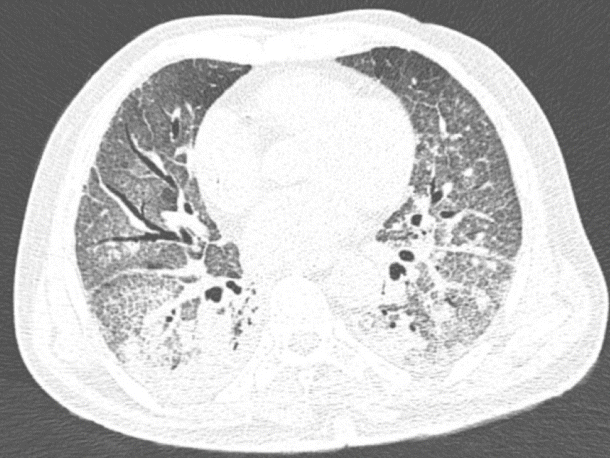
Fig. 2.
The esophagogastroduodenoscopy demonstrated a giant ulcer in (A) the greater curvature, (B) fundus, and (C) antrum.
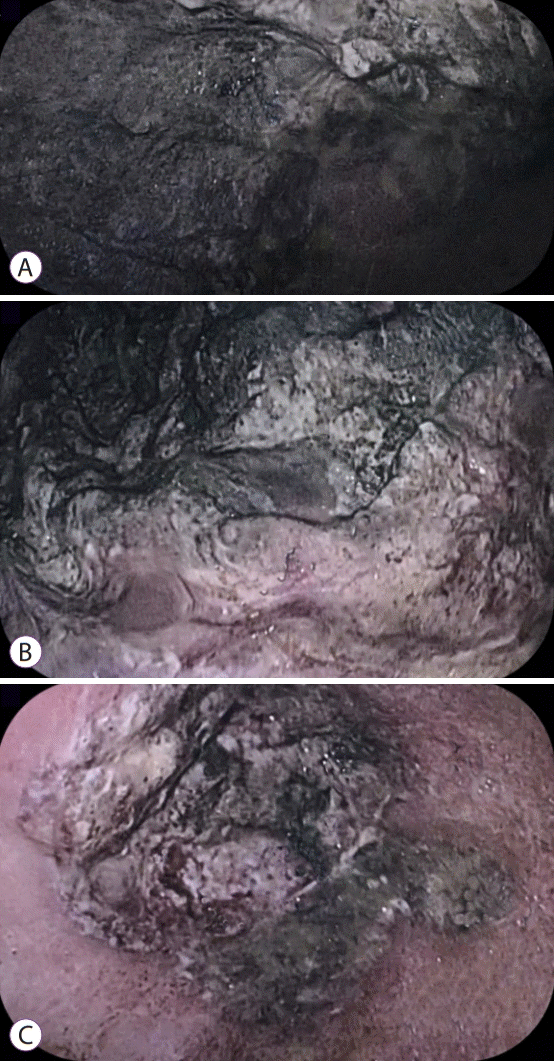
Fig. 3.
(A) The gastric border and base with necrotic fibrinoid debris. The fungus structures are visible at a low-power view (arrows). Hematoxylin and eosin staining, original magnification ×100. (B) Presence of broad, irregular, non-septate hyphae of mucormycosis (arrows). Hematoxylin and eosin staining, original magnification ×400. (C) Hyphae stained with periodic acid-Schiff stain (arrow), original magnification ×400. (D) Note the hyphae with typical 90-degree angle branching (arrow). Grocott’s methenamine staining, original magnification ×400.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download