Technical tips for puncture
The PD is either accessed with a 22-G or 19-G needle. The advantage of using a 22-G needle is that it becomes easy to puncture even a fibrotic pancreas or a stiff MPD (<5 mm). In fact, Matsunami et al. reported that the reason for their high technical success rate with EUS-PD, despite the small targeted MPD (median MPD diameter 3.5 mm, range 1–14 mm), might be attributed to the use of a 22-G needle [
6]. However, a 22-G needle can only use a 0.018- or 0.021-inch guidewire, which would have problems in terms of fluoroscopic visibility and sufficient stiffness that can withstand subsequent treatment. Therefore, a 19-G needle, which can accommodate a 0.025- or 0.035-inch guidewire, would be an optimal choice to access the MPD unless the parenchyma of the pancreas is highly fibrotic. Indeed, a 19-G needle was preferred to a 22-G needle in previous reports. Itoi et al. described in detail the needles and the guidewires used in EUS-PD [
9,
10]. In their report, a 0.025-inch guidewire “VisiGlide” with an angled tip (Olympus Medical Systems, Tokyo, Japan) was recommended because it had a soft, highly flexible tip with outstanding radiopacity, clear endoscopic visibility, sufficient stiffness at the guidewire shaft, seeking ability for easy therapeutic instrument exchange, and less kinking. In addition, a 0.025-inch and 0.035-inch Jagwire (Boston Scientific, Marlborough, MA, USA), 0.025-inch, 0.032-inch, and 0.035-inch Radifocus (Terumo Co., Tokyo, Japan), 0.035-inch Tracer (Cook Medical, Winston-Salem, NC, USA) for a 19-G needle, 0.018-inch and 0.021-inch Radifocus (Terumo Co.), 0.021-inch Metro (Cook Medical), 0.018-inch Pathfinder (Boston Scientific), and 0.018-inch Roadrunner (Cook Medical) for a 22-G needle have also been reported as guidewires for EUS-PD (
Table 3). When the 0.018-inch guidewire or 0.021-inch guidewire is used as the first guidewire, replacement with a large caliber and stiffer guidewire is recommended for subsequent intervention [
9-
11].
Table 3.
Various Guidewires Used in Endoscopic Ultrasound-Guided Pancreatic Duct Drainage
|
Product name |
Size (inch) |
Manufacturer |
References |
|
For 19-G needle |
|
|
|
|
VisiGlide/VisiGlide2 |
0.025 |
Olympus Medical Systems, Tokyo, Japan |
[2, 5, 6, 9, 10, 12, 15, 18, 22, 29] |
|
Jagwire |
0.025, 0.035 |
Boston Scientific, Marlborough, MA, USA |
[3, 7, 8] |
|
Radifocus |
0.025, 0.032, 0.035 |
Terumo Co., Tokyo, Japan |
[9, 12, 15] |
|
Tracer |
0.035 |
Cook Medical, Winston-Salem, NC, USA |
[29] |
|
For 22-G needle |
|
|
|
|
Radifocus |
0.018, 0.021 |
Terumo Co. |
[9] |
|
Metro |
0.021 |
Cook Medical |
[6, 18] |
|
Pathfinder |
0.018 |
Boston Scientific |
[6, 15, 18] |
|
Roadrunner |
0.018 |
Cook Medical |
[4] |

With respect to the considered parameters for needle selection, the sharpness of the tip is also an important factor along with the needle size. A stiff parenchyma resulting from chronic pancreatitis would prevent a smooth puncture in the absence of a sharp tip. Dhir et al. noted that a curved needle is not suitable for EUS-PD because the PD is thinner than the bile duct [
12]. They preferred a Sono-tip (MediGlobe GmbH, Achenmuhle, Germany) for chronic pancreatitis cases due to its sharpness and bendability. In addition, it has been reported that needles such as the 22 G/19 G Echo Tip (Cook Medical), 19 G Expect (Boston Scientific), and 19 G EZ shot 2/3 (Olympus Medical Systems) were used for EUS-PD.
The puncture site for EUS-PD is either transgastric or transduodenal. When selecting the puncture site, it is necessary to comprehensively judge the puncture angle to the MPD, where the blood vessel passes, and the distance from the stenosis. A transduodenal approach from the “long position” might allow a better view of the MPD for puncture than the transgastric approach [
3,
13]. Moreover, intraoperative stability and ease of pushing the stent were also reported as advantages associated with the transduodenal approach. However, in a transduodenal puncture, it might be difficult to pass through the stenosis of the guidewire when the stenosis is in the pancreatic body. In addition, care should be taken for the gastroduodenal artery running near the puncture route from the duodenal bulb, as it can be injured due to the burning effects of electrocautery dilation [
13]. Although previous reports seem to indicate that there is currently no difference in the technical and clinical perspectives by either route, a substantial number of available studies had selected the transgastric route. Overall, both transgastric and transduodenal routes are acceptable, but it is better to attempt puncturing from a site with better conditions.
Technical tips for fistula dilation
In EUS-PD, dilation of the gastric wall, pancreatic parenchyma, and MPD is challenging. For subsequent stent deployment, this step should be attempted. To obtain successful fistula dilation, guidewire manipulation is also important. The guidewire should be advanced deep enough for the rigid part to reach inside the MPD to ensure safe procedures afterward. An important aspect of this step in the case of a fibrotic and hardened pancreas is to efficiently apply force in the same direction as the puncture. In order to achieve this, it is necessary to confirm that the shape of the scope is the same as that at the time of puncture on fluoroscopic imaging, while the guidewire is kept visible in the longitudinal direction on ultrasonographic imaging throughout the procedure.
Endoscopic devices for dilating the needle tract are mainly divided into two types, non-electrocautery, and electrocautery. Non-electrocautery devices include mechanical dilators and balloon dilators, whereas electrocautery devices are classified into non-coaxial type and coaxial type. The main dilation devices that are currently used in EUS-PD are summarized in
Table 4. Step-up dilation of the needle tract using dilation catheters or balloon catheters for bougies is usually performed up to the size sufficient for stenting [
10]. Itoi et al. mentions the use of a tapered catheter (endoscopic retrograde cholangiopancreatography [ERCP] catheter; MTW Co., Dusseldorf, Germany) for the first bougie, followed by a 5–7 Fr dilation catheter (Soehendra; Cook Medical) or balloon catheters or the Cysto-Gastro Set (Endo-Flex, Voerde, Germany) in the subsequent steps [
9,
10].
Table 4.
Dilation Devices for the Fistula in Endoscopic Ultrasound-Guided Pancreatic Duct Drainage
|
Device |
Product name |
Manufacturer |
References |
|
Non-electrocautery |
|
|
|
|
|
Mechanical dilator |
ERCP catheter |
MTW Co., Dusseldorf, Germany |
[9, 10, 12] |
|
RR-V220Q ERCP catheter |
Olympus Medical Systems, Tokyo, Japan |
[5] |
|
ProForma ERCP catheter |
ConMed Endoscopic Technologies, Utica, NY, USA |
[8, 22] |
|
Sohendola stent retriever |
Cook Medical, Winston-Salem, NC, USA |
[8, 9, 10, 15, 22] |
|
ES dilator |
Zeon Medical Co., Tokyo, Japan |
[18] |
|
Balloon dilator |
PD-SS6F180C |
Gadelius Medical, Tokyo, Japan |
[12] |
|
REN |
KANEKA Medics, Osaka, Japan |
[12, 14, 18] |
|
Titan |
Cook Medical |
[8, 22] |
|
Hurricane RX |
Boston Scientific, Marlborough, MA, USA |
[6, 7, 8, 12, 15, 18, 22, 29] |
|
Sterling |
Boston Scientific |
[4] |
|
Maxforce TTS |
Boston Scientific |
|
|
Electrocautery |
|
|
|
|
Non-Coaxial |
NeedleCut 3V |
Olympus Medical Systems |
[9] |
|
MicroKnife |
Boston Scientific |
[29] |
|
Coaxial |
Fine 025 |
Medico’s HIRATA Inc., Osaka, Japan |
[13, 16] |
|
Cyst-Gastro Set |
Endo-Flex, Voerde, Germany |
[3, 6, 9, 10, 12, 15, 18] |
|
Cystotome CST10 |
Cook Medical |
|
|
Will’s high frequency ring knife |
MTW Co. |
[7] |

With respect to the use of balloon catheters, we have previously shown the usefulness of a fine-gauge balloon catheter REN (KANEKA Medics, Osaka, Japan) for EUS-PD (
Fig. 3) [
14]. This catheter was characterized by a 3 Fr ultra-tapered tip and coaxial guidewire followability. Hayat et al. reported a retrospective study of EUS-PD using a small-caliber balloon (Sterling; Boston Scientific) in eight patients [
4]. This balloon catheter was originally designed for use in angioplasty and had only a 4 Fr diameter. The technical success rate with dilation using only this small-diameter balloon was 88% (7/8). In the unsuccessful case, the device could not cross the pancreatic parenchyma, and a small leakage in the duct occurred that did not require any additional intervention. The author concluded that the caliber balloon from Sterling offered a safe and atraumatic alternative without the use of a cautery device.
 | Fig. 3.REN (KANEKA Medics, Osaka, Japan). This balloon catheter is characterized by a 3 Fr ultra-tapered tip and coaxial guidewire followability. 
|
There are some reports on the use of mechanical dilation or electrocautery dilation on a case-specific basis. In a retrospective study evaluating 44 EUS-PD cases, one of the following was used as the dilation method for EUS-PD: a Soehendra stent retriever, a Hurricane RX (Boston Scientific), or a Cysto-Gastro Set [
15]. The authors noted that a Hurricane RX or Cysto-Gastro Set was used for antegrade transmural tract dilation, while the Soehendra stent retriever or Cysto-Gastro Set was used for retrograde transmural tract dilation after rendezvous, respectively. The technical success in this study was 84.1% (37/44), and the most common cause for failure of the procedure was reported to be an inability to puncture the MPD because of severe fibrosis and calcification in the pancreas. Immediate adverse events were seen in 22.7% (
n=10) of the cases, and they were sufficiently mild to be managed conservatively.
There is a report of a case in which dilation of the needle tract using fine diathermic dilation (Fine 025; Medico’s HIRATA Inc., Osaka, Japan;
Fig. 4) was successful after dilation with a mechanical dilator (ES dilator; Zeon Medical Co., Tokyo, Japan;
Fig. 5) failed due to hard pancreatic parenchyma [
16]. The distal end of a Fine 025 is only 3 Fr and contains a metal tip. This diathermic dilator is coaxial with a guidewire and can be useful for tract dilation in a severely fibrotic pancreas. In addition to its high ability to rupture the tissue, the Fine 025 is expected to have the potential to reduce damage to the surrounding regions because of a thin electrode on the tip.
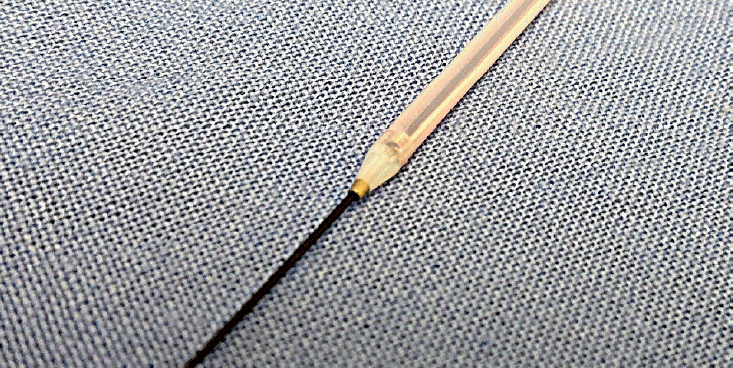 | Fig. 4.Fine 025 (Medico’s HIRATA Inc., Osaka, Japan). The distal end of this diathermic dilator is only 3 Fr, and it contains a metal tip. This catheter is coaxial with a guidewire and can be useful for tract dilation in a severely fibrotic pancreas. 
|
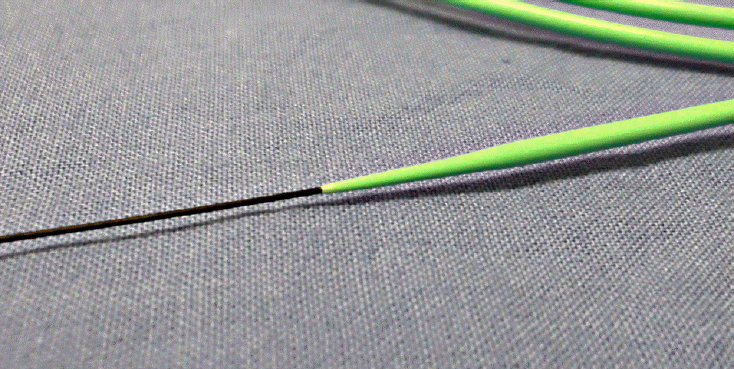 | Fig. 5.ES dilator (Zeon Medical Co., Tokyo, Japan). This mechanical dilator can be pushed to a greater degree and exhibits only a small difference in the diameter of the inner lumen and the guidewire. 
|
As mentioned above, a mechanical dilator is usually used first to dilate the puncture fistula [
5,
8-
10]. Although cautery-assisted devices may prove to be more efficacious when pancreatic parenchyma is hardened from fibrosis [
17-
19], their use is limited and is often positioned as a subsequent method when dilation with mechanical devices fail. This is mainly because of the risk of adverse events associated with cautery devices. Indeed, many experts recommend the use of electrocautery devices only as a rescue technique when other approaches fail [
17,
20-
22]. Dilation with electrocautery catheters in EUS-PD has been reported to be capable of causing acute and late “burn-effects” around the tract, leading to severe adverse events including pancreatitis, pancreatic juice leakage, bleeding, and perforation [
9]. A study comparing the safety of a mechanical dilator and an electrocautery dilator in patients undergoing EUS-hepaticogastrostomy (
n=49) and EUS-PD (
n=15) showed that there was no significant difference in the rate of adverse events (16.1% vs. 27.2%,
p=0.48) between the two groups [
18]. Comparing the reports of adverse events in the literature, procedure-related bleeding was observed only in the electrocautery dilator group (0% vs. 18.2%,
p=0.04). Therefore, it has been suggested that a mechanical dilator was more suitable for interventional EUS, particularly in patients taking antithrombotic drugs or when the blood vessels were located near the puncture line. Matusnami et al. reported severe bleeding that required transcatheter arterial embolization after dilation using an electrocautery dilator in EUS-PD [
6]. Therefore, they warned that tract dilation using an electrocautery dilator may cause unexpected bleeding due to a burn effect even with the use of Doppler mode, in order to avoid injuring the intervening blood vessels under EUS guidance.
There has been no detailed study that determined the criteria for the selection of non-coaxial or coaxial electrocautery in EUS-PD. Park et al. studied the predictors of adverse events in 57 patients who underwent EUS-guided biliary drainage (EUS-BD) with transluminal stenting and showed that the use of a cautery-assisted device was an independent predictor of adverse events (odds ratio [OR], 12.4;
p=0.01) [
23]. The electrocautery device used in that study was a non-coaxial needle knife. In contrast, a comparative study of mechanical dilation and coaxial electrocautery dilation in EUS-guided pancreatic fluid collection drainage showed no significant difference in the occurrence of adverse events [
19]. Free air was seen as an immediate adverse event in only one case in the non-electrocautery group (1/28). Khashab et al. also evaluated the adverse events in patients who underwent EUS-BD (
n=121) [
24]. In that study, non-coaxial and coaxial electrocautery devices were separately analyzed, and only non-coaxial electrocautery was found to be independently associated with adverse events (OR, 3.95;
p=0.03). Especially, when the scope is in an acute angulation position, the non-coaxial type needle-knives are oriented tangentially, leading to undesired incisions and adverse events [
22]. Such a scenario can be prevented by maintaining a degree of tension over the guidewire that would keep the needle-knife catheter in the same plane as the guidewire, as it exits the scope. Even in EUS-PD, the non-coaxial type may require more attention than the coaxial type when using electrocautery devices for dilation.
Non-electrocautery devices also have a risk of adverse events. Catheter dilation was reported to be associated with an axial force, which can lead to the separation of tissue planes during bougie advancement [
20]. In addition, balloon dilation increased the risk of perforation, leakage, and bleeding with its radial force [
20]. According to a study (
n=28) that utilized a mechanical dilator including an ES dilator, a 7-Fr tapered catheter, and a 4-mm balloon dilator for EUS-BD, adverse events occurred in four patients (14.3%, 4 bile peritonitis) [
25], and all patients recovered with conservative drug treatment. We also evaluated the feasibility and safety of balloon catheter REN in EUS-BD (
n=20) [
26]. Adverse events were seen in 15% (3/20; self-limited abdominal pain
n=2, peritonitis
n=1) of cases in that study. All the cases were mild, and they did not require any additional intervention.
In conclusion, there is still no clear consensus on which devices are to be used first during EUS-PD. However, it seems that non-cautery-assisted devices might be recommended as the primary dilation device as per the available reports. Prospective trials aimed at standardizing the technique for performing dilation in EUS-PTS are necessary to design conclusive models for such procedures.
Technical tips for stent deployment
Most stents that are reported to be placed transmurally in EUS-PD are plastic stents [
4-
6,
15]. Both straight and pig tail stents with diameters of 5 to 7 Fr have been frequently used. When plastic stents are selected for EUS-PTS, an “all-in-one stent” is recommended to avoid insertion failure [
9]. The “all-in-one stent” has the advantage of being able to return to the state before stent release when the length of the stent is inappropriate at the time of placement of the stent, or when stent advancement is impossible across the tract. This is possible because of the presence of a string between the stent and the delivery system. As a result of this advantage. different stent placement or additional tract dilation can be carried out when stent insertion is difficult. “Ring drainage” (i.e., gastropancreaticoduodenostomy) has been described as an important technique for preventing the migration in EUS-PTS [
17,
21]. This procedure can be performed only when the stent is advanced so that the distal end terminates in the small bowel anterogradely, and the proximal tip of the stent rests within the gastric lumen. Ring drainage allows future stent exchange easily and reduces the risk of stent-induced ductal change. Originally, transmural plastic stent deployment for EUS-PD might have had lesser migration into the abdominal cavity than EUS-guided hepaticogastrostomy because the pancreas and the gastric wall are in close contact [
13]. Particularly in cases in which the PD is not very dilated, plastic stents are more suitable than metallic stents. The rates of technical success and adverse events of EUS-PTS using plastic stents were reported to range from 80.9% to 100% and from 12.5% to 26.7%, respectively [
3,
5,
6,
15,
27]. These immediate adverse events included abdominal pain, peritonitis, bleeding, pancreatic fluid collection, MPD leakage, and perforation. Matsunami et al. evaluated the usefulness of the 7 Fr single pigtail type plastic stent (CX-T stent, TYPE IT; Gadelius Medical Co., Tokyo, Japan;
Fig. 6), which has a total length of 20 cm and an effective length of 15 cm, for EUS-PTS [
6]. The author discussed the length of the stent and stated that 15 cm was a suitable size for this stent in EUS-PTS. Although the distal part of the stent was placed in the MPD, and two-thirds of the stent was retained on the stomach side, the long length was effective in preventing stent migration into the PD. A pigtail anchor together with its four flanges also prevented unnecessary migration. Indeed, there was no stent migration in that study. In addition, no pancreatic juice leakage was seen, probably because the stent had a side hole at the proximal and distal ends, but not in the middle part of the stent.
 | Fig. 6.TYPE IT (Gadelius Medical Co., Tokyo, Japan). This 7 Fr single pigtail type plastic stent has a total length of 20 cm and an effective length of 15 cm. The length and a pigtail anchor together with its four flanges are effective in preventing stent migration. 
|
To overcome the disadvantage of transmural plastic stenting procedures in EUS-PD such as stent migration, stent placement failure, pancreatic juice leakage, and stent occlusion, a fully covered self-expandable metal stent (FCSEMS) was used as an alternative. Uncovered SEMSs are contraindicated because of the risk of pancreatic juice leakage and the difficulty in removing or replacing them due to tissue ingrowth [
17]. A large diameter FCSEMS might have the advantage of effective drainage and easy re-intervention compared to plastic stents [
13]. In addition, the tamponade effect of FCSEMS may prevent pancreatic juice leakage and reduce the bleeding risk associated with electrocautery tract dilation. Moreover, FCSEMS may provide extended stent patency, fewer repeat interventions, and shorter hospital stays [
2,
28]. Oh et al., used a modified 6- or 8-mm FCSEMS (M.I. tech, Seoul, Korea) with blunt ends and anchoring flaps for EUS-PTS after failed ERCP in 25 patients with painful obstructive pancreatitis [
29]. The anchoring flaps of the FCSEMS with antimigration properties were designed to prevent proximal and distal migration. Stent placement was achieved in all patients with no severe or major adverse events. No stent occlusion, migration, and pancreatitis caused by cross-stream blockage were seen. Stent-induced ductal change after stent removal was also not observed. Oh et al. also assessed the outcomes of EUS-PTS using FCSEMS for pancreaticojejunal anastomosis strictures following Whipple procedures [
2]. Technical and clinical success were also both achieved in all the 20 cases, as well, in the above-mentioned study by the same team. Early adverse events with EUS-PTS using FCSEMS developed in three patients (15%, 3/20), and all of them had mild self-limited abdominal pain. The favorable success and adverse event rates in the study were not inferior to those of plastic stents found in the previous study, but the evidence for EUS-PTS using FCSEMSs is limited. Indeed, Oh et al., stated that the results obtained using the FCSEMS might not be extrapolated to other FCSEMSs with different diameters and radial forces [
2]. However, further evaluation of FCSEMS use, especially a comparative study with a plastic stent, is necessary, Thus, EUS-PTS with an FCSEMS for MPD obstruction may be safe and feasible.
The indications for a lumen-apposing metal stent (LAMS), which was originally designed to provide solid anchorage between nonadherent luminal structures, have widened. It is now attributed to have effective roles in pancreatic cyst drainage, biliary drainage, and gastrojejunostomy [
30]. The structure of this stent is characterized by a fully covered dumbbell-shaped short stent made up of braided nitinol wire, which makes it possible to prevent tract leakage, migration, and tissue ingrowth and allows removability and trans-stent interventional procedures. Will et al. reported a case of EUS-PTS using LAMS (AXIOS; Boston Scientific) for a patient with recurrent episodes of severe chronic pancreatitis and retention of fluid in the PD against a background of pancreatolithiasis with ERCP failure [
7]. Since LAMS cannot be placed unless the PD is considerably dilated, the indications for using LAMS in EUS-PTS seem to be limited.
Long-term outcomes of endoscopic ultrasound-guided pancreatic transmural stenting
The long-term efficacy and safety of EUS-PTS have been studied, as well as the early and mid-term outcomes. Matsunami et al. reported in detail the long-term clinical outcomes after EUS-PTS, using plastic stents [
6]. For 25 patients who underwent successful EUS-PTS and could be followed-up, stent exchange was planned every 3–4 months during the year after initial stent placement, unless spontaneous dislodgement occurred. Four patients were stent-free 1 year after EUS-PTS as evinced from the improvement in their symptoms. Twelve patients who received regular stent exchange had no recurrence of symptoms. Further, in contrary to transpapillary PD, stenting under ERCP guidance was achieved in three patients. The leading late adverse events were stent dislodgement (24%, 6/25), followed by recurrent pancreatitis (8%, 2/25), within a median time of 23 months (range, 6–44 months). In this study, long-term clinical success was achieved in 92% (23/25) of the total cases. In another study that evaluated EUS-PTS with plastic stents (
n=44), delayed adverse events such as stent blockage and spontaneous stent migration were seen in 12 (31%) and five (13%) subjects, respectively [
15]. In all patients who suffered from delayed adverse events, stent exchanges were attempted, which were carried out successfully in all the 17 cases. On the other hand, as per the long-term outcome of EUS-PTS with FCSEMS that was studied by Oh et al. [
2], 20 patients underwent EUS-guided transmural FCSEMS placement and were followed-up for a median of 27.2 months. During the follow-up period, late adverse events associated with the stent were seen in five (25%) patients, including FCSEMS occlusion (
n=1), asymptomatic stent fracture at the gastric end (
n=3), and stent migration (
n=1). In particular, the patient with FCSEMS occlusion required stent revision. Stent fractures at the gastric end were reported to occur in patients who underwent an 8-cm stent deployment at 12.4, 21.9, and 46.4 months after EUS-PTS, respectively. Stent exchanges were performed in these three patients, although the remnant stents seemed to be functioning well as assessed by computed tomography imaging. Pancreatitis due to upstream or side-branch obstruction by FCSEMSs did not develop even in the long-term follow-up period. These reports suggest that the long-term outcomes of EUS-PTS are as favorable as the short and mid-term outcomes. Further evidence supporting the use of EUS-PTS with a longer follow-up period is required to establish the efficacy of the procedure.




 PDF
PDF Citation
Citation Print
Print







 XML Download
XML Download