1. ASGE Technology Committee, Wang A, Banerjee S, et al. Wireless capsule endoscopy. Gastrointest Endosc. 2013; 78:805–815.

2. Lim YJ, Lee OY, Jeen YT, et al. Indications for detection, completion, and retention rates of small bowel capsule endoscopy based on the 10-year data from the Korean capsule endoscopy registry. Clin Endosc. 2015; 48:399–404.

3. Pennazio M, Spada C, Eliakim R, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2015; 47:352–376.

4. Enns RA, Hookey L, Armstrong D, et al. Clinical practice guidelines for the use of video capsule endoscopy. Gastroenterology. 2017; 152:497–514.
5. Rondonotti E, Villa F, Mulder CJ, Jacobs MA, de Franchis R. Small bowel capsule endoscopy in 2007: indications, risks and limitations. World J Gastroenterol. 2007; 13:6140–6149.

6. Kwack WG, Lim YJ. Current status and research into overcoming limitations of capsule endoscopy. Clin Endosc. 2016; 49:8–15.

7. Kim SH, Yang DH, Kim JS. Current status of interpretation of small bowel capsule endoscopy. Clin Endosc. 2018; 51:329–333.

8. Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000; 405:417.

9. Höög CM, Bark L, Arkani J, Gorsetman J, Broström O, Sjöqvist U. Capsule retentions and incomplete capsule endoscopy examinations: an analysis of 2300 examinations. Gastroenterol Res Pract. 2012; 2012:518718.

10. Ou G, Shahidi N, Galorport C, Takach O, Lee T, Enns R. Effect of longer battery life on small bowel capsule endoscopy. World J Gastroenterol. 2015; 21:2677–2682.

11. Shamsudhin N, Zverev VI, Keller H, et al. Magnetically guided capsule endoscopy. Med Phys. 2017; 44:e91–e111.

12. Nam SJ, Lee HS, Lim YJ. Evaluation of gastric disease with capsule endoscopy. Clin Endosc. 2018; 51:323–328.

13. Liu L, Towfighian S, Hila A. A review of locomotion systems for capsule endoscopy. IEEE Rev Biomed Eng. 2015; 8:138–151.

14. Swain P. The future of wireless capsule endoscopy. World J Gastroenterol. 2008; 14:4142–4145.

15. Swain P, Toor A, Volke F, et al. Remote magnetic manipulation of a wireless capsule endoscope in the esophagus and stomach of humans (with videos). Gastrointest Endosc. 2010; 71:1290–1293.
16. Keller J, Fibbe C, Volke F, et al. Inspection of the human stomach using remote-controlled capsule endoscopy: a feasibility study in healthy volunteers (with videos). Gastrointest Endosc. 2011; 73:22–28.

17. Rey JF, Ogata H, Hosoe N, et al. Blinded nonrandomized comparative study of gastric examination with a magnetically guided capsule endoscope and standard videoendoscope. Gastrointest Endosc. 2012; 75:373–381.

18. Jiang X, Qian YY, Liu X, et al. Impact of magnetic steering on gastric transit time of a capsule endoscopy (with video). Gastrointest Endosc. 2018; 88:746–754.

19. Rey JF, Ogata H, Hosoe N, et al. Feasibility of stomach exploration with a guided capsule endoscope. Endoscopy. 2010; 42:541–545.

20. ASGE Technology Committee. Magnets in the GI tract. Gastrointest Endosc. 2013; 78:561–567.
21. Bang S, Park JY, Jeong S, et al. First clinical trial of the “MiRo” capsule endoscope by using a novel transmission technology: electric-field propagation. Gastrointest Endosc. 2009; 69:253–259.

22. Rahman I, Pioche M, Shim CS, et al. Magnetic-assisted capsule endoscopy in the upper GI tract by using a novel navigation system (with video). Gastrointest Endosc. 2016; 83:889–895.e1.
23. Lien GS, Liu CW, Jiang JA, Chuang CL, Teng MT. Magnetic control system targeted for capsule endoscopic operations in the stomach--design, fabrication, and in vitro and ex vivo evaluations. IEEE Trans Biomed Eng. 2012; 59:2068–2079.
24. Lien GS, Wu MS, Chen CN, Liu CW, Suk FM. Feasibility and safety of a novel magnetic-assisted capsule endoscope system in a preliminary examination for upper gastrointestinal tract. Surg Endosc. 2018; 32:1937–1944.

25. Ching HL, Hale MF, Sidhu R, Beg S, Ragunath K, McAlindon ME. Magnetically assisted capsule endoscopy in suspected acute upper GI bleeding versus esophagogastroduodenoscopy in detecting focal lesions. Gastrointest Endosc. 2019; 90:430–439.

26. Ching HL, Hale MF, Kurien M, et al. Diagnostic yield of magnetically assisted capsule endoscopy versus gastroscopy in recurrent and refractory iron deficiency anemia. Endoscopy. 2019; 51:409–418.

27. Ciuti G, Donlin R, Valdastri P, et al. Robotic versus manual control in magnetic steering of an endoscopic capsule. Endoscopy. 2010; 42:148–152.

28. Liao Z, Duan XD, Xin L, et al. Feasibility and safety of magnetic-controlled capsule endoscopy system in examination of human stomach: a pilot study in healthy volunteers. J Interv Gastroenterol. 2012; 2:155–160.

29. Zou WB, Hou XH, Xin L, et al. Magnetic-controlled capsule endoscopy vs. gastroscopy for gastric diseases: a two-center self-controlled comparative trial. Endoscopy. 2015; 47:525–528.

30. Liao Z, Hou X, Lin-Hu EQ, et al. Accuracy of magnetically controlled capsule endoscopy, compared with conventional gastroscopy, in detection of gastric diseases. Clin Gastroenterol Hepatol. 2016; 14:1266–1273. e1.
31. Qian YY, Zhu SG, Hou X, et al. Preliminary study of magnetically controlled capsule gastroscopy for diagnosing superficial gastric neoplasia. Dig Liver Dis. 2018; 50:1041–1046.

32. Zhao AJ, Qian YY, Sun H, et al. Screening for gastric cancer with magnetically controlled capsule gastroscopy in asymptomatic individuals. Gastrointest Endosc. 2018; 88:466–474.e1.

33. Hu J, Wang S, Ma W, Pan D, Sun S. Magnetically controlled capsule endoscopy as the first-line examination for high-risk patients for the standard gastroscopy: a preliminary study. Scand J Gastroenterol. 2019; 54:934–937.

34. Xie M, Qian Y, Cheng S, Wang L, Shen R. Magnetically guided capsule endoscopy in pediatric patients with abdominal pain. Gastroenterol Res Pract. 2019; 2019:7172930.

35. Liao Z, Zou W, Li ZS. Clinical application of magnetically controlled capsule gastroscopy in gastric disease diagnosis: recent advances. Sci China Life Sci. 2018; 61:1304–1309.

36. Jiang B, Qian YY, Pan J, et al. Second-generation magnetically controlled capsule gastroscopy with improved image resolution and frame rate: a randomized controlled clinical trial (with video). Gastrointest Endosc. 2020; 91:1379–1387.
37. McAlindon ME, Ching HL, Yung D, Sidhu R, Koulaouzidis A. Capsule endoscopy of the small bowel. Ann Transl Med. 2016; 4:369.

38. Gan T, Wu JC, Rao NN, Chen T, Liu B. A feasibility trial of computer-aided diagnosis for enteric lesions in capsule endoscopy. World J Gastroenterol. 2008; 14:6929–6935.

39. Rondonotti E, Spada C, Adler S, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) technical review. Endoscopy. 2018; 50:423–446.

40. McCarthy J, Minsky ML, Rochester N, Shannon CE. A proposal for the Dartmouth summer research project on artificial intelligence, August 31, 1955. AI Mag. 2006; 27:12–14.
41. Le Berre C, Sandborn WJ, Aridhi S, et al. Application of artificial intelligence to gastroenterology and hepatology. Gastroenterology. 2020; 158:76–94.e2.

42. Suzuki K. Overview of deep learning in medical imaging. Radiol Phys Technol. 2017; 10:257–273.

43. Choi J, Shin K, Jung J, et al. Convolutional neural network technology in endoscopic imaging: artificial intelligence for endoscopy. Clin Endosc. 2020; 53:117–126.

44. Alaskar H, Hussain A, Al-Aseem N, Liatsis P, Al-Jumeily D. Application of convolutional neural networks for automated ulcer detection in wireless capsule endoscopy images. Sensors (Basel). 2019; 19:1265.

45. Leenhardt R, Vasseur P, Li C, et al. A neural network algorithm for detection of GI angiectasia during small-bowel capsule endoscopy. Gastrointest Endosc. 2019; 89:189–194.

46. Tsuboi A, Oka S, Aoyama K, et al. Artificial intelligence using a convolutional neural network for automatic detection of small-bowel angioectasia in capsule endoscopy images. Dig Endosc. 2020; 32:382–390.

47. Aoki T, Yamada A, Aoyama K, et al. Automatic detection of erosions and ulcerations in wireless capsule endoscopy images based on a deep convolutional neural network. Gastrointest Endosc. 2019; 89:357–363.e2.

48. Aoki T, Yamada A, Kato Y, et al. Automatic detection of blood content in capsule endoscopy images based on a deep convolutional neural network. J Gastroenterol Hepatol. 2020; 35:1196–1200.

49. Ding Z, Shi H, Zhang H, et al. Gastroenterologist-level identification of small-bowel diseases and normal variants by capsule endoscopy using a deep-learning model. Gastroenterology. 2019; 157:1044–1054.e5.
50. Rondonotti E, Koulaouzidis A, Karargyris A, et al. Utility of 3-dimensional image reconstruction in the diagnosis of small-bowel masses in capsule endoscopy (with video). Gastrointest Endosc. 2014; 80:642–651.
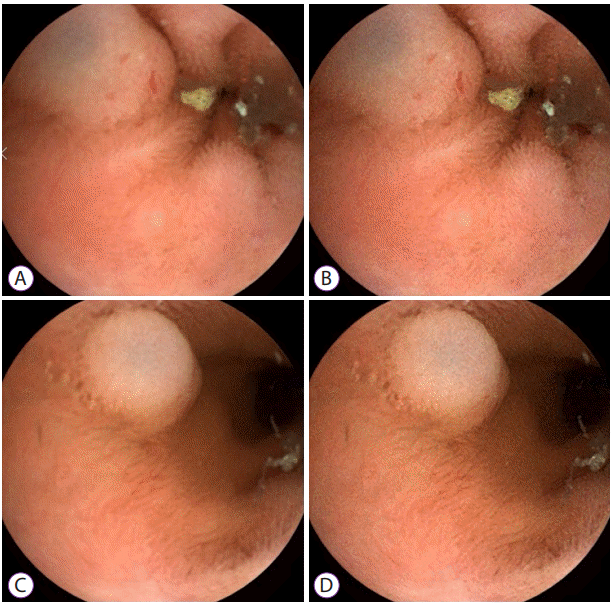
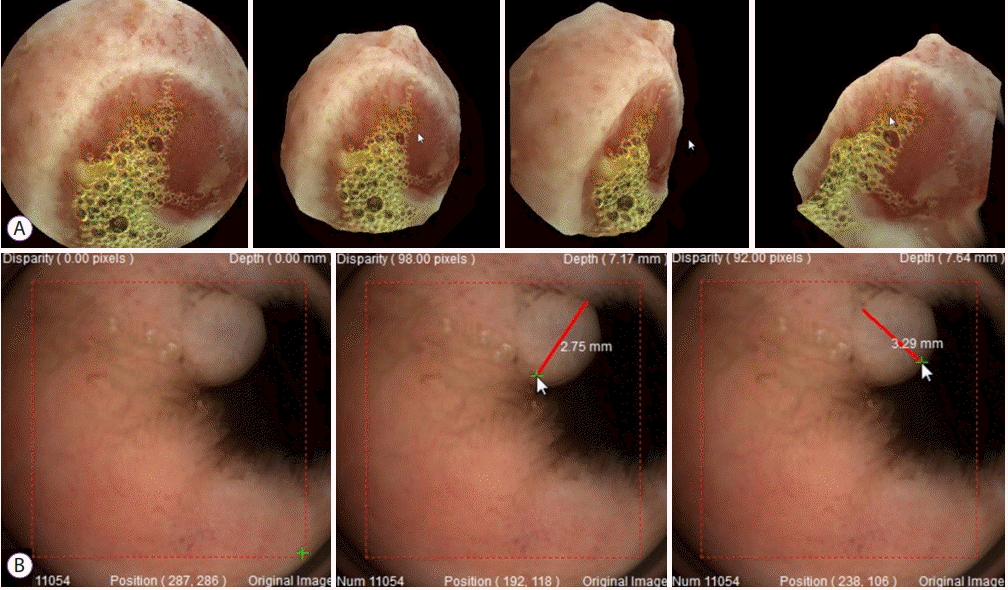
51. Nam SJ, Lim YJ, Nam JH, et al. 3D reconstruction of small bowel lesions using stereo camera-based capsule endoscopy. Sci Rep. 2020; 10:6025.

52. Gora MJ, Sauk JS, Carruth RW, et al. Tethered capsule endomicroscopy enables less invasive imaging of gastrointestinal tract microstructure. Nat Med. 2013; 19:238–240.

53. Cotter J, Magalhães J, de Castro FD, et al. Virtual chromoendoscopy in small bowel capsule endoscopy: new light or a cast of shadow? World J Gastrointest Endosc. 2014; 6:359–365.

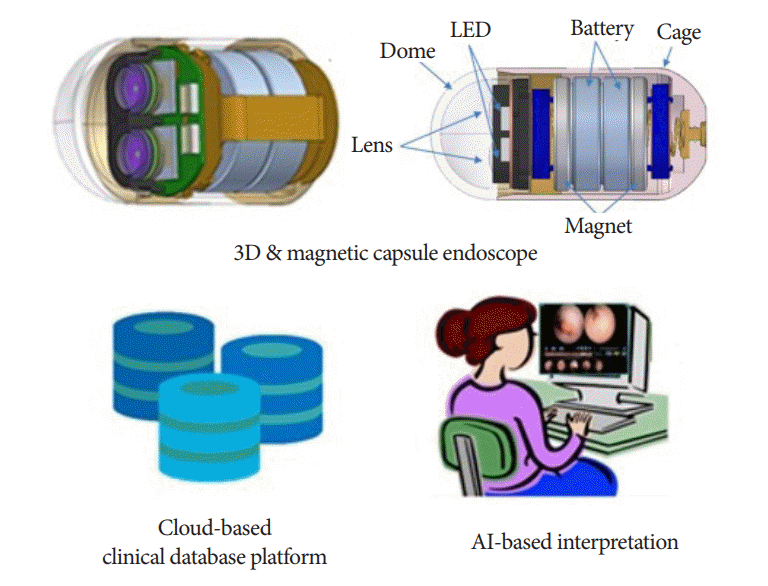
54. Koulaouzidis A, Iakovidis DK, Yung DE, et al. Novel experimental and software methods for image reconstruction and localization in capsule endoscopy. Endosc Int Open. 2018; 6:E205–E210.

55. Kim HM, Kim YJ, Kim HJ, et al. A pilot study of sequential capsule endoscopy using MiroCam and PillCam SB devices with different transmission technologies. Gut Liver. 2010; 4:192–200.

56. Subramanian V, Ragunath K. Advanced endoscopic imaging: a review of commercially available technologies. Clin Gastroenterol Hepatol. 2014; 12:368–376.e1.





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download