Abstract
Background/Aims
Three-dimensional (3D) flexible endoscopy, a new imaging modality that provides a stereoscopic view, can facilitate endoscopic hand suturing (EHS), a novel intraluminal suturing technique. This ex-vivo pilot study evaluated the usefulness of 3D endoscopy in EHS.
Methods
Four endoscopists (two certified, two non-certified) performed EHS in six sessions on a soft resin pad. Each session involved five stitches, under alternating 3D and two-dimensional (2D) conditions. Suturing time (sec/session), changes in suturing time, and accuracy of suturing were compared between 2D and 3D conditions.
Results
The mean suturing time was shorter in 3D than in 2D (9.8±3.4 min/session vs. 11.2±5.1 min/session) conditions and EHS was completed faster in 3D conditions, particularly by non-certified endoscopists. The suturing speed increased as the 3D sessions progressed. Error rates (failure to grasp the needle, failure to thread the needle, and puncture retrial) in the 3D condition were lower than those in the 2D condition, whereas there was no apparent difference in deviation distance.
Endoscopic hand suturing (EHS) is a novel suturing method that allows optimal and secure intraluminal suturing [1,2]. In EHS for mucosal defects, the mucosal rim of the defect is continuously and linearly sutured using a transorally delivered needle and suture. Although EHS is expected to be a promising technique for the development of various advanced therapeutic endoscopies, it requires advanced technical skills and significant experience in puncturing the mucosa using the needle, optimally grasping the needle, and tightening the thread using a flexible endoscope; therefore, how to efficiently obtain these necessary skills is an issue to be addressed.
Technological advances in laparoscopic surgery have enabled the creation of stereoscopic videos or three-dimensional (3D) images from conventional two-dimensional (2D) video images and have led to favorable results in the clinical settings [3,4]. Recently, 3D flexible endoscopy has also been described and is expected to be useful for both endoscopic diagnosis [5,6] and treatment [7-9]. Thus, we hypothesized that 3D endoscopy can reliably enhance EHS performance compared with conventional 2D endoscopy and verified the usefulness of 3D endoscopy in EHS in an ex-vivo study.
Four endoscopists (two certified and two non-certified) without prior EHS experience, were selected and provided brief instruction by an EHS expert. A certified endoscopist was defined as a doctor with a license of specialist as authorized by the Japan Gastroenterological Endoscopy Society. Each endoscopist performed EHS alternately under 2D or 3D conditions, with six sessions per condition sequentially, with one assistant having minimal EHS experience. The participants had not received any prior training. Written informed consent was provided by the participants prior to study initiation.
The endoscopy system used was identical to that used in surgery, which mainly included an EVIS EXERA III Video System Center, equipped with a 3D Visualisation Unit (CV190; Olympus Co., Ltd., Tokyo, Japan) and EVIS EXERA III Video Processor (3DV-190; Olympus). In these systems, switching from the 2D to the 3D mode and vice versa can be accomplished by pushing a button on the head of the endoscope. The 3D images were visualized using a 3D monitor and 3D glasses, as described elsewhere [7-9].
We prepared eight urethane pads designed for surgical skin suturing training (AS ONE Co., Osaka, Japan). Each pad could accommodate six sutures, and each suturing area had 10 markings: five each on the right and left rows (Fig. 1A). The interval between successive markings was set at 5 mm. One pad was used per one condition (2D or 3D); thus, each participant used two pads. The pads were placed in a multipurpose training box for flexible endoscopy using EHS.
EHS was performed using a GIF-Y0080 3D scope (Olympus) equipped with a prototype flexible needle holder (Olympus) and 15-cm, 3–0 absorbable barbed V-Loc 180 (VLOCL0604) sutures (Covidien, Mansfield, MA, USA), as described elsewhere [1,2]. Briefly, a needle with a knot at the tail was inserted from the markings on the right row and pulled out from the markings on the left row (Supplementary video 1), and the sutures were placed continuously and linearly from the distal to the proximal side (Fig. 1B). Any slack in the thread was appropriately tightened by directly grasping the thread with the needle holder. All procedures were recorded as videos.
While reviewing the videos, we estimated the suturing time (sec/session) and number of errors (failure to grasp the needle, failure to grasp the thread, and puncture repetition). Next, the sutured pad was used to assess deviation distance, measured as the interval between the actual insertion/exit site and the marking where the needle should have passed.
The primary outcome measure was suturing time under two conditions (2D and 3D). The suturing time for each endoscopist, changes in suturing time, deviation distance, and error rate were also evaluated. Finally, subjective assessments among the endoscopists (degree of learning, interest, satisfaction, self-confidence, difficulty, and eye strain) after the EHS procedure were also performed using a visual analog scale (VAS) scale, with scores ranging from 1 to 4 points (1: very bad, 2: bad, 3: good, 4: very good).
Because this phase I pilot study lacked a statistically calculated sample size, the obtained data were shown with 95% confidence intervals (CIs). We analyzed the suturing time and error rates between the 2D and 3D groups using nonparametric statistic methods (Mann–Whitney U tests). Analyses were performed using the SPSS statistical software package version 25 (IBM Co., New York, NY, USA).
Of 48 total sessions by four participants in the 2D and 3D conditions, EHS was successfully completed in all except for one 2D session (47/48 sessions; 98%) due to unexpected thread disconnection. We omitted data from this incomplete session during analyses and did not add a compensatory session to avoid a possible learning effect from the incomplete session.
The mean suturing time was 9.8±3.4 min/session (95% CI, 8.4–11.2) in 3D, which was shorter than in that in 2D (11.2±5.1 min/session [95% CI, 8.9–13.4]), but this difference was not significant (p=0.23) (Fig. 2A). No participants showed a significant increase in their suturing speed in 3D compared to that in 2D (Fig. 2B). Similarly, no significant difference in suturing time was seen between the 3D and 2D conditions between certified and non-certified endoscopists (10.1 min/session in 3D vs. 9.6 min/session in 2D, p=0.98; non-certified, 9.5 min/session in 3D vs. 12.5 min/session in 2D, p=0.27) (Fig. 2C).
Fig. 3 shows the changes in suturing time. All participants tended to suture faster under both conditions as their experience with EHS increased during the course of this study. Specifically, the suturing time decreased with less variation in 3D than in 2D (R=0.65 in 3D and 0.56 in 2D) (Fig. 3A, B). Furthermore, the mean suturing time in the first and last three sessions were 13.2 and 9.3 min/session in 2D (p=0.10) and 11.3 and 8.4 min/session in 3D (p=0.045), with the latter showing a significant reduction in the suturing time (Fig. 3C).
There was no apparent difference in deviation distance between the two conditions (Table 1). However, the error rates (failure to grasp the needle, failure thread the needle, and puncture retrial) in the 3D group were lower than those in the 2D group (Table 1). Concerning subjective assessments, we found no significant differences between the 2D and 3D groups in the degree of learning (3 vs. 3.25, p=0.39), interest (2.75 vs. 3.25, p=0.39), satisfaction (2.5 vs. 3.25, p=0.097), self-confidence (2.5 vs. 3.25, p=0.097), difficulty (2.25 vs. 2.5, p=0.67), and eye strain (3 vs. 2.75, p=0.39) (Table 1).
This ex-vivo pilot study investigated the usefulness of 3D endoscopy in EHS, a representative advanced endoscopic technique. The results demonstrated that 3D endoscopy facilitated EHS compared to 2D endoscopy as it reduced suturing time. 3D endoscopy facilitates stereoscopic depth recognition; therefore, endoscopists can easily grasp, set the direction, and insert the needle at an optimal angle in EHS.
Sakata et al. [10] demonstrated that all laparoscopic tasks were faster in 3D than in 2D, with an approximately 30% reduction in completion time and that 3D endoscopy appeared to contribute to time savings during endoscopic submucosal dissection in ex-vivo porcine models [8,9]. We observed a 12% reduction in suturing time in EHS (from 11.2 to 9.8 min/session) in the present study, although the difference was not statistically significant. The lack of significant reduction may be due to the insufficient number of sessions and the difficulty of the task tested, as suturing involves tissue apposition requiring at least two stitches and tightening of the thread; continuing this procedure under flexible endoscopy is complicated and technically demanding. Furthermore, the artificial situation by the use of urethane pads may prevent smooth suturing compared to other conditions such as isolated/live porcine stomachs or even clinical settings. Therefore, the 12% reduction in suturing time under this condition is acceptable to demonstrate the advantage of 3D in EHS. In one endoscopist, the mean suturing time was shorter in 2D than that in 3D because the suture was accidentally cut when a slack part of the suture was pulled too strongly in one 3D session, which required extra time to retry the suturing.
3D endoscopy appears particularly helpful in novice endoscopists. In general, repetitive training in endoscopic procedures accelerates the improvement of these skills, which is also applicable to difficult and complicated techniques including EHS. In this study, by using 3D, even non-certified endoscopists performed EHS in comparable times to those of certified endoscopists. This finding implies that 3D may facilitate technically challenging procedures for less experienced endoscopists. Further study is needed to investigate the potential of 3D endoscopy to make advanced procedures accessible to endoscopists with various degrees of technical skill.
Our data also showed a significant reduction in suturing time in the last three sessions of 3D endoscopy, suggesting a meaningful learning effect and implying that endoscopists can more efficiently acquire suturing skills using 3D viewing compared with 2D viewing when EHS is repeatedly performed. A previous report also indicated that robotic surgery improves surgical performance and learning, especially with 3D viewing [11]. Taken together, these results indicate that the skills required for advanced endoscopic techniques can be obtained more quickly using 3D endoscopy; however, further assessment is needed.
Although the error rates were lower under the 3D condition than those under the 2D condition, we could not determine the improvement in the technical accuracy of EHS due to the lack of clear evidence. The lower error rates may have been because of better depth perception in the 3D view compared to that in the 2D view, as the former simulates an environment similar to the real world.
We evaluated the subjective assessment of endoscopists using the VAS scale (learning, interest, satisfaction, self-confidence, difficulty, eye strain) and observed no significant differences between the two conditions. However, the relatively high scores for satisfaction and self-confidence in the 3D groups compared to those in the 2D groups may imply that 3D endoscopy is more accessible to endoscopists than conventional endoscopy.
The present study has several limitations. First, all participants always started the procedure with the 2D condition and performed suturing under either 2D or 3D alternately per session (1st, 2D; 2nd, 3D; 3rd, 2D; 4th, 3D, etc.). Therefore, a training effect by performing 2D first could be a bias in this study. Second, this was an ex-vivo pilot study under artificially provided conditions with a small number of sessions and participants. A prospective comparative study in clinical settings with a large number of endoscopists and a statistically calculated sample size based on this preclinical pilot study is necessary to validate these preliminary results on the usefulness of 3D endoscopy in EHS.
In conclusion, our data suggest that 3D endoscopy may efficiently contribute to fast and accurate EHS and may be particularly helpful for novice endoscopists. Further clinical investigations are necessary to validate these results.
Notes
Conflicts of Interest: The flexible needle holder and scissors forceps used in this study were complimentarily provided by Olympus Co., Ltd.
Author Contributions
Conceptualization: Osamu Goto
Data curation: Jun Omori, Kazutoshi Higuchi, Takamitsu Umeda, Naohiko Akimoto, Masahiro Suzuki
Formal analysis: JO
Funding acquisition: OG
Investigation: JO, Kumiko Kirita, Eriko Koizumi, Hiroto Noda, Teppei Akimoto
Methodology: OG
Project administration: JO, OG
Supervision: OG, Mitsuru Kaise, Katsuhiko Iwakiri
Validation: OG, KK, EK, HN, TA
Writing-original draft: JO
Writing-review&editing: JO, MK, KI
Supplementary Material
Video 1. Endoscopic hand suturing in this ex-vivo study. The soft urethane pad is continuously sutured with a barbed suture and a flexible needle holder, from right to left, and from the distal to the proximal direction. A loosened part of the string is appropriately tightened (https://doi.org/10.5946/ce.2019.207.v001).
REFERENCES
1. Goto O, Sasaki M, Ishii H, et al. A new endoscopic closure method for gastric mucosal defects: feasibility of endoscopic hand suturing in an ex vivo porcine model (with video). Endosc Int Open. 2014; 2:E111–E116.

2. Goto O, Sasaki M, Akimoto T, et al. Endoscopic hand-suturing for defect closure after gastric endoscopic submucosal dissection: a pilot study in animals and in humans. Endoscopy. 2017; 49:792–797.

3. Becker H, Melzer A, Schurr MO, Buess G. 3-D video techniques in endoscopic surgery. Endosc Surg Allied Technol. 1993; 1:40–46.
4. Fergo C, Burcharth J, Pommergaard HC, Kildebro N, Rosenberg J. Three-dimensional laparoscopy vs 2-dimensional laparoscopy with high-definition technology for abdominal surgery: a systematic review. Am J Surg. 2017; 213:159–170.

5. Nomura K, Kaise M, Kikuchi D, et al. Recognition accuracy using 3D endoscopic images for superficial gastrointestinal cancer: a crossover study. Gastroenterol Res Pract. 2016; 2016:4561468.

6. Nomura K, Kaise M, Kikuchi D, et al. Recognition accuracy of tumor extent using a prototype 3D endoscope for superficial gastric tumor: an ex vivo crossover study. Endosc Int Open. 2018; 6:E652–E658.

7. Kikuchi D, Kaise M, Nomura K, et al. Feasibility study of the three-dimensional flexible endoscope in endoscopic submucosal dissection: an ex vivo animal study. Digestion. 2017; 95:237–241.

8. Nomura K, Kikuchi D, Kaise M, et al. Comparison of 3D endoscopy and conventional 2D endoscopy in gastric endoscopic submucosal dissection: an ex vivo animal study. Surg Endosc. 2019; 33:4164–4170.

9. Akizue N, Matsumura T, Maruoka D, et al. Novel three-dimensional imaging system may facilitate gastric endoscopic submucosal dissection procedure: an ex vivo animal study. Endosc Int Open. 2018; 6:E1431–E1435.

Fig. 1.
Ex-vivo settings of endoscopic hand suturing. (A) Locations of the markings on the urethane pad. Five markings at 5-mm intervals are linearly placed in both the right and left rows. (B) The pad after suturing. The suture needle is inserted from the marking point on the right row and pulled out from the marking point on the left row; this suturing is repeated five times per session.
Fig. 2.
Suturing time during endoscopic hand suturing (EHS). (A) Comparison of suturing time (min/session) between two-dimensional (2D) and three-dimensional (3D) endoscopy showing a shorter time in 3D. (B) Comparison of suturing time for each endoscopist. (C) Among non-certified participants, EHS is completed faster in 3D than in 2D. Bar: 95% confidence interval.
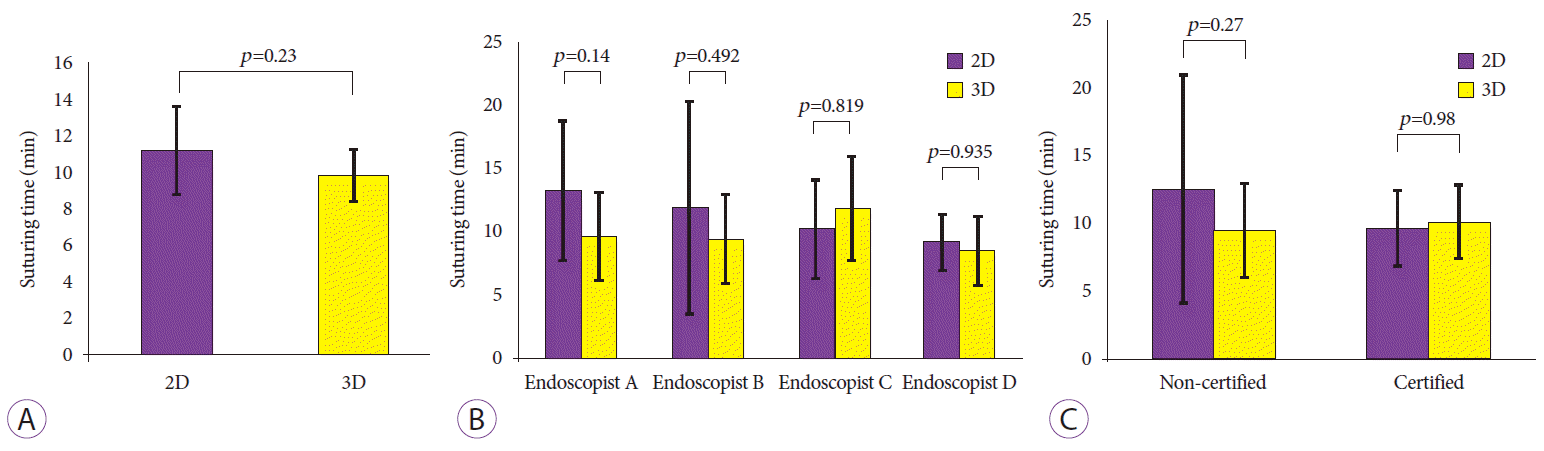
Fig. 3.
Changes in suturing time. (A) Under two-dimensional (2D) conditions, the suturing time per session tends to decrease as the study proceeded. (B) The time also decreases in the three-dimensional (3D) condition, although the magnitude of change is less than that seen under 2D conditions. (C) Comparison of suturing times in the former and latter halves of the suture sessions showing a reduced time in the 3D condition. Bar: 95% confidence interval.
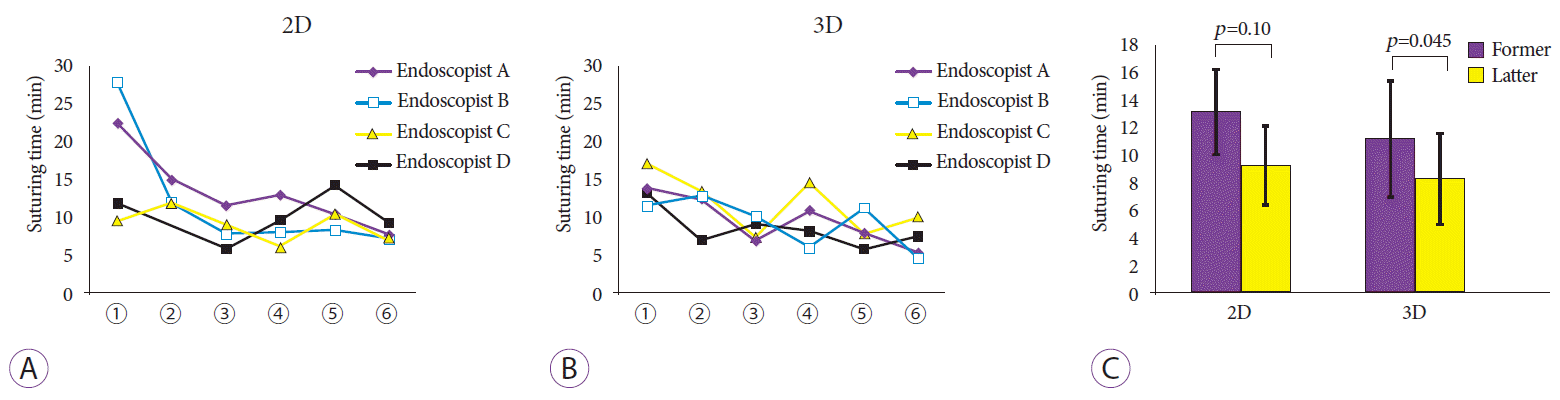
Table 1.
Outcomes of Technical Accuracy in Endoscopic Hand Suturing




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download