INTRODUCTION
Endoscopic bariatric and metabolic therapies (EBMTs) have recently been developed as an alternative treatment option for obesity. Compared to lifestyle intervention and pharmacotherapy, patients who undergo EBMTs generally achieve more significant weight loss, while maintaining a lower risk profile than those who undergo bariatric surgery. Additionally, for some patients with class I and II obesity who do not meet the criteria for surgery and those with class III obesity who do not want to undergo surgery, EBMTs may represent a feasible option for the treatment of obesity and potentially other metabolic comorbiditie [
1-
6].
Aspiration therapy (AT) is one of the available EBMTs that utilizes a device called the AspireAssist (Aspire Bariatrics, King of Prussia, PA, USA). The device was approved by the Food and Drug Administration in 2016 for long-term use in conjunction with lifestyle therapy (LT) for people with body mass index (BMI) of 35–55 kg/m
2 [
7]. Accordingly, in this review, AT refers to AT in conjunction with LT. The device consists of the A-tube, which is a 26 Fr percutaneous endoscopic gastrostomy tube with a 15-cm fenestrated intragastric drainage catheter, a skin port, which connects to the external end of the A-tube and is normally closed to prevent gastric leakage, and a detachable connector, which connects with the skin port to allow aspiration of gastric contents. Participants aspirate approximately 30% of ingested calories at 30 minutes after meals, in addition to undergoing LT. After 115 uses, which is equivalent to 5 to 6 weeks of therapy, the connector locks and can no longer be used. Patients are required to see a practitioner who will provide a new connector as well as reinforce the importance of LT. It has been estimated that less than 80% of the weight loss is due to the aspiration of calories. The remaining 20% or more of the weight loss is thought to be due to a reduction in food intake. This is likely attributable to multiple factors including (1) food particles having to be less than or equal to 5 mm to fit through the A-tube, which likely leads to significantly longer chewing of food and reduced calorie consumption as a result; (2) increased water consumption to allow liquid gastric contents to flow out of the A-tube, which likely increases the sense of satiety without additional calories; and (3) the visibility of the gastric aspirate. Patients report that less healthy food options have an unappealing appearance on aspiration leading to a reduction in the consumption of those foods [
8].
To date, several studies have reported the effectiveness of AT at inducing clinically significant weight loss. However, the effect of AT on obesity-related comorbidities remains unclear. This systematic review and meta-analysis aims to evaluate the changes in obesity-related comorbidities following AT. Additionally, the long-term effect of AT on weight profiles and its pooled serious adverse events (SAEs) will be assessed.
DISCUSSION
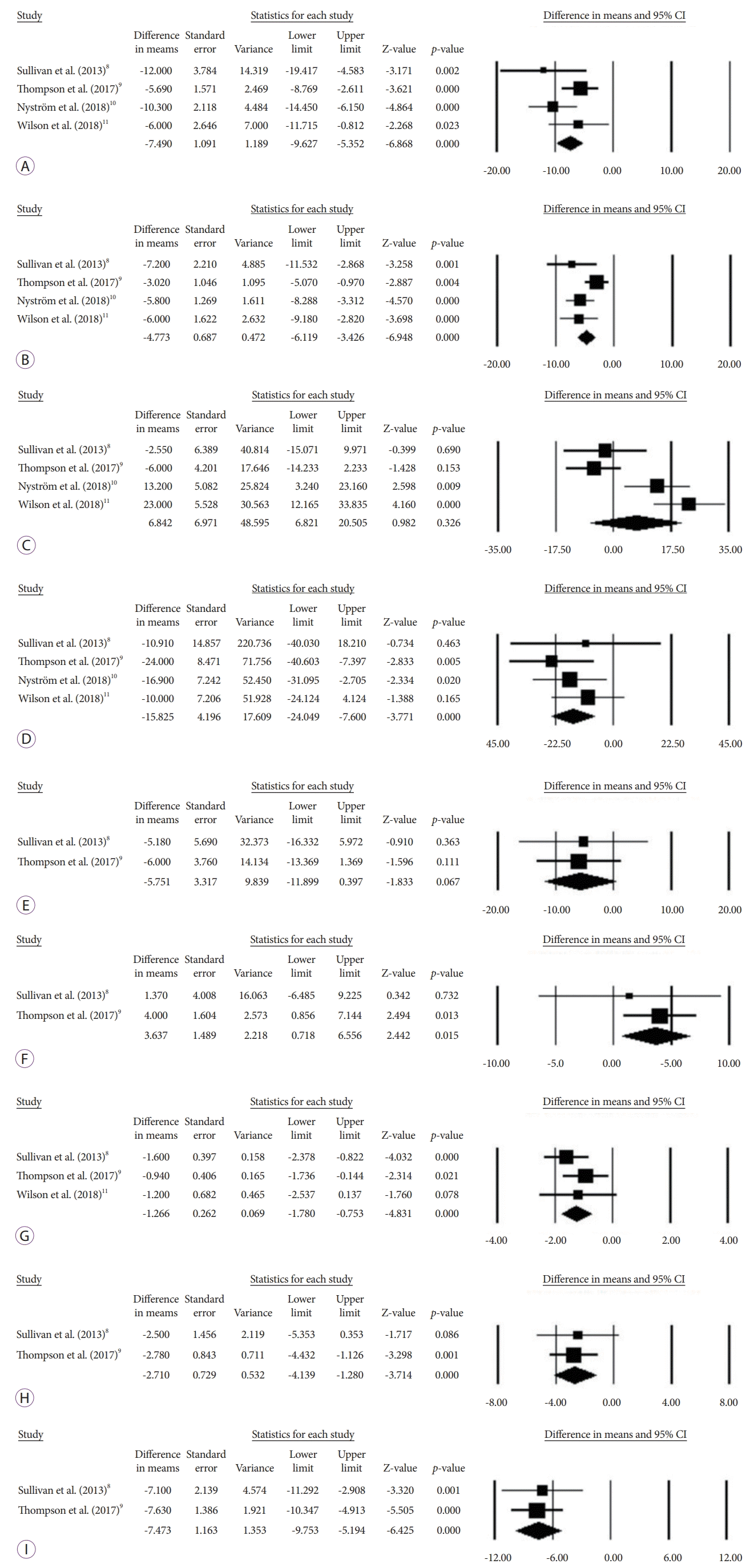
This systematic review and meta-analysis is the first to evaluate the effect of AT on metabolic comorbidities. Our study demonstrates that AT is associated with significant improvement in major metabolic outcomes including SBP, DBP, TG, HDL, LDL, HbA1c, AST, and ALT. Additionally, weight loss of approximately 17% to 19% of the baseline weight can be achieved and maintained up to at least 4 years following initiation of therapy, with an acceptable risk profile demonstrated by a 4.1% pooled SAE rate.
Obesity is a leading cause of preventable death in the U.S. that results in as much as 47% more life-years lost than tobacco use [
13]. Following obesity, other top modifiable risk factors in decreasing order include diabetes, tobacco use, hypertension, and hyperlipidemia, 3 of which (diabetes, hypertension, and hyperlipidemia) are direct and indirect adverse consequences of the increased body fat and adiposopathic dysfunction seen in patients with obesity. As a result, the goal of most obesity treatments is to improve metabolic outcomes, which will ultimately lead to a decrease in mortality risk.
Previous meta-analyses have shown the beneficial effects of other EBMTs including intragastric balloons (IGBs) and duodenal jejunal bypass liner (DJBL) on obesity-related comorbidities. Specifically, Popov et al. conducted a meta-analysis of 40 studies with 5,668 patients who underwent IGB placement [
14]. The study showed significant improvement in SBP by 9.1 mm Hg, DBP by 4.6 mm Hg, TG by 33.4 mg/dL, HbA1c by 0.6%, AST by 3 U/L, and ALT by 9 U/L at the time of IGB removal at 6 months, when data from observational studies were pooled [
14]. More recently, Jirapinyo et al. conducted a meta-analysis on 14 studies with 412 patients with obesity and concomitant T2DM who underwent DJBL [
15]. At the time of DJBL removal at 12 months, patients experienced a significant improvement in T2DM with a decrease in HbA1c by 1.3%, as well as improvement in insulin resistance and several gut hormones that control hunger, satiety, and glucose hemostasis [
15].
To the best of our knowledge, the present study is the first to elucidate the impact of AT on metabolic outcomes. Specifically, this study demonstrates that the chemical surrogates of the major metabolic conditions including hypertension, hyperlipidemia, T2DM, and NAFLD all significantly improve following AT. In contrast to prior studies on AT that focused primarily on its effect on weight loss, our study aimed to evaluate the effect of AT on metabolic comorbidities as the primary outcome. Additional data were obtained from every available study, allowing this meta-analysis to be sufficiently powered to detect changes in comorbidities, in contrast to prior studies that showed variable improvement in metabolic outcomes.
This improvement in metabolic functions is likely related to the amount and type of weight loss following AT. Specifically, a prospective study on 40 volunteers demonstrated that subjects who experienced 5%, 11%, and 16% TWL had preferentially and disproportionately lost more intra-abdominal fat (9%, 23%, and 30%, respectively) and intra-hepatic fat (13%, 52%, and 65%, respectively), which likely explained the stepwise metabolic benefits of weight loss at different levels [
16]. Additionally, different tissues responded to different degrees of weight loss. While 5% TWL significantly decreased glucose, insulin, TG, ALT, and leptin, only after 16% TWL did plasma free fatty acid, C-reactive protein, and adiponectin improve [
16]. This evidence likely explains the findings that 1) at a TWL of 2%–5%, there was an improvement in HbA1c, SBP, and TG; 2) at a TWL of 5%–10%, there was an improvement in DBP and HDL; and 3) at a TWL of at least 10%, there was an improvement in non-alcoholic steatohepatitis histologic features [
17-
19]. In this study, AT was associated with 17.8% TWL at 1 year, which was above the 16% threshold for improvement in both metabolic/cardiovascular risk factors and inflammatory markers and likely explains the improvement in the chemical surrogates of all major obesity-related comorbidities shown in the study.
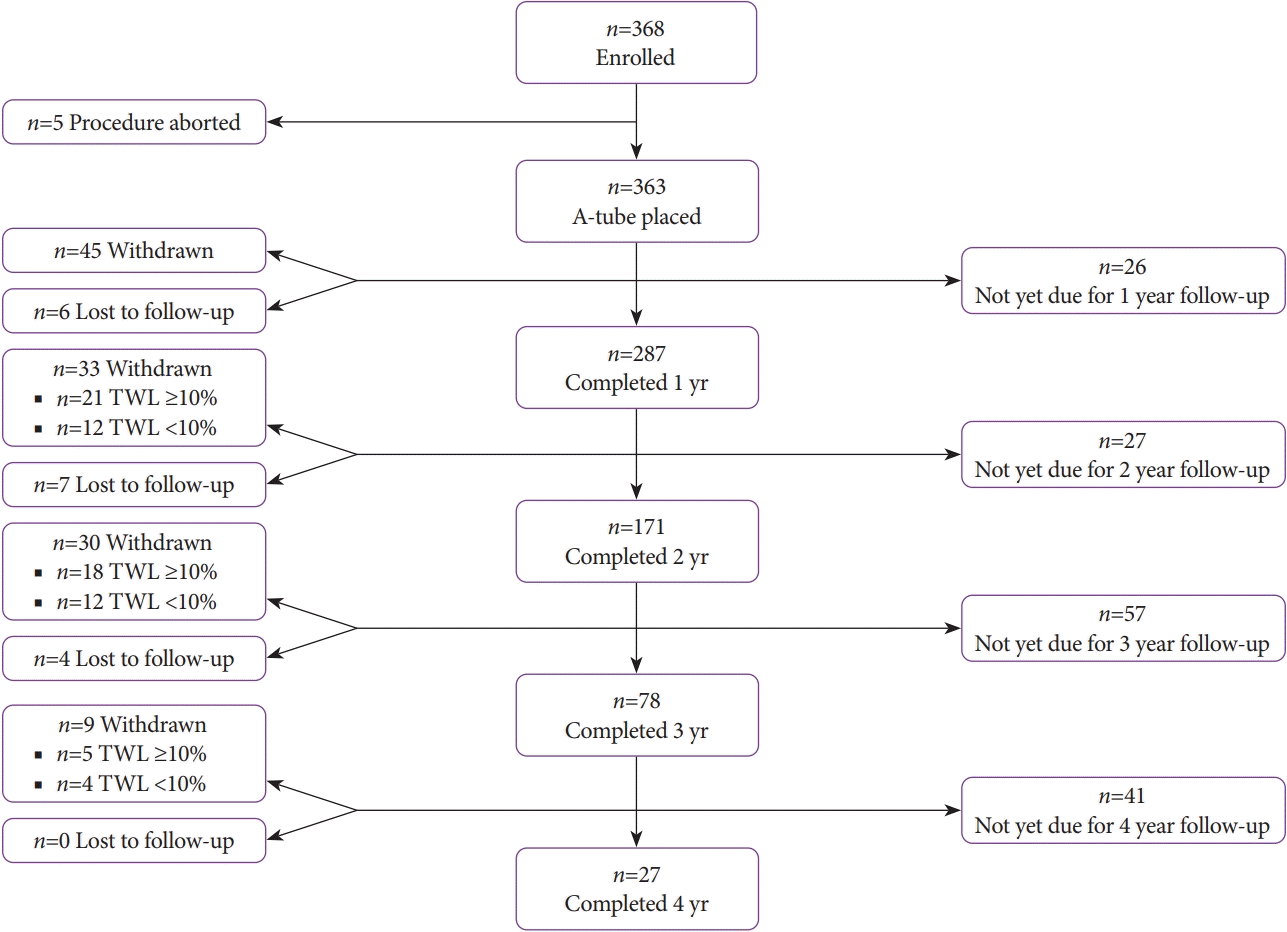
A challenge of most obesity treatments is long-term weight loss maintenance. While several definitions have been proposed to define successful weight loss maintenance, it is generally considered to be a sustained weight loss of 5% to 10% of baseline weight in at least 1 year as recommended by the 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults [
20,
21]. In our study, at 4 years following the initiation of AT, patients maintained their significant weight loss of 18.6% of their baseline weight, meeting the definition of successful weight loss maintenance. This suggests that the mechanisms of AT in combination with self-directed non-high-intensity LT (<14 lifestyle program sessions/6 months) were able to counteract physiological and cognitive adaptations favoring weight regain. In terms of the weight trends yielded by other treatment modalities, these results place AT closer to bariatric surgery than to lifestyle intervention and/or pharmacology. Specifically, a recent systematic review on the outcome of lifestyle intervention showed that weight loss reached its peak at 6 months after initiation of treatment. Without an active maintenance program, 50% of patients had returned to their original weight at 5 years [
22]. In contrast, a recent study showed that patients who underwent Roux-en-Y gastric bypass experienced 35% TWL at 2 years and were able to maintain their weight at 28% TWL and 26.9% TWL at 6 and 12 years, respectively [
23].
In our study, a subgroup analysis of different age groups showed no difference in weight outcomes following AT. In fact, at 4 years, the older patients experienced significantly greater weight loss (31.5% TWL) compared to the younger group (16.9% TWL). There have been controversies regarding the outcomes of bariatric and metabolic surgery in elderly patients, commonly defined as aged 55 years and older in most bariatric literature. Specifically, some studies showed that younger bariatric patients had better weight loss and comorbidity outcomes compared to the elderly group [
24,
25], although the data remained conflicted [
26,
27]. Therefore, it has been suggested that bariatric surgical indications in elderly patients should be carefully considered. Given the minimal invasiveness of AT, acceptable safety profile of upper endoscopy, and favorable outcomes of AT in the elderly [
28-
30], it is possible that AT may have a broad application for those in this patient population who meet the BMI criteria.
Treatment with AT is accompanied by AEs, some of which are serious. In our study, the pooled SAE rate was 4.1%, which is comparable to that of other EBMTs and within the risk threshold of ≤5% set by the ASGE/ASMBS Task Force on Endoscopic Bariatric Therapy [
31,
32]. The most common SAE was buried bumper, which is a known complication of percutaneous endoscopic gastrostomy tubes that is thought be due to excessive tension on the internal bumper against the gastric wall. The buried bumpers were treated with removal of the A-tube, temporary replacement with a 20-Fr percutaneous endoscopic gastrostomy tube, followed by replacement with an A-tube. Persistent gastrocutaneous fistula following A-tube removal occurred, especially after 2 years of AT. All but 2 cases (1.4% of all A-tubes removed) were successfully closed with endoscopic interventions.
This study has some limitations. First, the number of included studies was relatively small. To account for this, conference abstracts that met the a priori inclusion criteria were included in the analysis. Additionally, we were able to obtain additional data from all studies to make the analysis robust and sufficiently powered to detect beneficial changes in all obesity-related comorbidities. Another limitation is the fact that most of the published studies on AT were retrospective observational studies with a proportion of patients who were not yet due for follow-ups at the respective time points. Nevertheless, the primary outcome of this meta-analysis, which was the change in comorbidities at 1 year, was derived from a pool of almost 300 patients, making this a robust analysis. Another limitation is that each included study focused on weight loss as the primary outcome, with changes in comorbidities as a secondary outcome. These changes in metabolic parameters were reported for all subjects who underwent AT, including those with and without comorbidities at baseline. Additionally, the present study did not take into account the changes in anti-hypertensive, anti-hyperlipidemic, and anti-glycemic medications throughout the study period. Therefore, the changes in metabolic outcomes reported in this study were likely conservative and possibly underestimated the true effect of AT on obesity-related comorbidities.
In summary, this systematic review and meta-analysis suggests that AT is associated with significant improvement in hypertension, hyperlipidemia, T2DM, and NAFLD in patients with class II and III obesity. Moreover, substantial weight loss was experienced by this population, which persisted for at least 4 years in a subgroup of patients who continued to use the therapy appropriately. Given its simplicity and minimally invasive nature, AT may improve access to treatment in patients with obesity and concomitant metabolic comorbidities.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download