Abstract
Background/Aims
The purpose of this study was to investigate the risk factors and long-term clinical outcomes of non-curative resection (NCR) in a large-scale patient population.
Methods
We retrospectively analyzed the clinical data of 3,094 patients who underwent endoscopic submucosal dissection (ESD) of early gastric cancer from March 2005 to March 2018 at 13 institutions in Korea. We analyzed the risk factors for NCR and the survival between patients with curative resection and those with NCR with no additional treatment.
Results
The NCR rate was 21.4% (661/3,094). In multivariate regression analysis, the risk factors affecting NCR with ESD were old age, undifferentiated tumor, tumor location in the upper body, tumor size ≥2 cm, and presence of an ulcer. In Cox proportional hazard regression analysis, tumor size ≥2 cm, submucosal invasion, positive horizontal margin, and lymphovascular invasion were risk factors for local recurrence. In Kaplan-Meier analysis, there was no statistically significant difference in the overall survival between the two groups (log-rank p=0.788). However, disease-specific survival was significantly lower in the NCR group (log-rank p=0.038).
Gastric cancer is the fourth most common cancer and has the second highest cancer-related mortality rate worldwide [1,2]. Early detection and early treatment can significantly reduce mortality [3,4]. In Korea, with the increase in individual health examinations and the expansion of the national cancer screening program that provides upper gastrointestinal endoscopy every 2 years for people aged >40 years, the number of patients diagnosed with early gastric cancer (EGC) has increased [2,5]. Traditionally, surgical resection was the standard treatment for gastric cancer; however, in recent years, endoscopic resection has become widely used. At present, endoscopic submucosal dissection (ESD) is accepted as a standard treatment for gastric cancer in patients with a negligible risk of lymph node or distant metastasis [6-9].
Endoscopic resection of EGC is indicated on the basis of tumor differentiation, size, ulceration, and invasion depth, and its goal is “curative resection” (CR) [10]. In cases of non-curative resection (NCR), additional treatment is needed because of local recurrence and lymph node metastasis, for which the standard treatment is gastrectomy with lymphadenectomy [11-13]. However, for reasons such as an increased proportion of elderly patients owing to the increase in average life expectancy, concomitant diseases, poor general health, or a patient’s refusal to undergo surgery, patients with NCR may be treated with redo-ESD, argon plasma coagulation, or careful observation without further treatment [14-16].
Although several studies have reported the long-term clinical outcomes of NCR in patients with EGC treated with ESD, studies on large-scale populations are lacking [17-19]. The aims of this retrospective multicenter study were to investigate the risk factors associated with NCR and to assess the long-term clinical outcomes after NCR with no additional treatment in a large-scale patient population.
We performed a retrospective multicenter study at 13 Korean institutions by using ESD registry data collected under the supervision of the ESD research group of the Korean Society of Gastrointestinal Endoscopy. The study protocol adhered to the ethical principles of the Declaration of Helsinki and was approved by the institutional review board of each institution (2019-05-035).
We retrospectively analyzed the clinical data of 3,929 patients aged >20 years who underwent endoscopic resection of EGC from March 2005 to March 2018 at 13 institutions in Korea. We excluded patients who met any of the following exclusion criteria: (1) history of previous endoscopic resection (n=101), (2) history of previous abdominal surgery for stomach cancer (n=14), (3) EGC treated with endoscopic mucosal resection (n=237), (4) surgery performed immediately after ESD because of NCR or meeting the expanded criteria (n=115), or (5) loss to follow-up or <6 months of follow-up (n=368). A total of 3,094 patients were finally enrolled (Fig. 1).
Among patients diagnosed with EGC by using standard endoscopy, ESD was performed in those who met the indications for endoscopic resection. All patients were sedated using midazolam and/or propofol with cardiopulmonary monitoring. The target lesion was identified and marking dots were placed circumferentially 2 mm outside the lesion to determine the ESD range. Thereafter, a submucosal solution, such as saline or sodium hyaluronate with epinephrine, was injected into the submucosal layer to lift it off the muscle layer and a circumferential mucosal incision was made outside the marking dots with an electrosurgical knife. The submucosal layer was then dissected with an IT-knife or an IT-knife2 while performing hemostasis on any oozing vessel or on vessels exposed both during and after the procedure.
The endoscopically resected specimens were sectioned at 2-mm intervals and stained with hematoxylin and eosin after fixation in 10% formalin and embedding in paraffin for histopathological evaluation. At each institution, a pathologist evaluated the specimens for histopathological type, invasion depth, horizontal and vertical margins, ulcerations, and lymphovascular invasion according to the Japanese classification of gastric carcinoma [20]. Differentiated adenocarcinoma included tubular adenocarcinoma and papillary adenocarcinoma, whereas undifferentiated adenocarcinoma included poorly differentiated adenocarcinoma, signet ring cell carcinoma, and mucinous adenocarcinoma. Tumoral infiltration of the submucosa (SM) was subclassified as SM1 (<500 μm from the muscularis mucosa) or SM2 (≥500 μm from the muscularis mucosa).
According to the Japanese gastric cancer treatment guidelines, the indications for CR are as follows: en bloc resection, tumor size ≤2 cm, histologically differentiated type, pT1a, negative horizontal margin (HM0), negative vertical margin (VM0), and no lymphovascular invasion. In addition, a resection was considered curative for the expanded indications when all of the following conditions were fulfilled: en bloc resection, HM0, VM0, no lymphovascular invasion with (1) tumor size >2 cm, differentiated, pT1a, no ulcerative findings; (2) tumor size ≤3 cm, differentiated, pT1a, ulcerative findings; (3) tumor size ≤2 cm, undifferentiated, pT1a, no ulcerative findings; and (4) tumor size ≤3 cm, differentiated, pT1b (SM1). Any resection that did not satisfy any of the above criteria (expanded indications) was considered NCR.
After ESD, regular follow-up was performed at 3 months, 6 months, and every year thereafter. In this study, local recurrence was defined as a recurrence of the index cancer at the site of ESD. Synchronous cancer was defined as a development of new cancer at a site other than the ESD site within 1 year after ESD. Metachronous cancer was defined as the development of new cancer beyond 1 year after ESD. Overall survival (OS) was defined as the period from the initial ESD until death due to any cause or last patient contact. Disease-specific survival (DSS) was defined as the period from the initial ESD until gastric cancer-related death or last patient contact. Disease-free survival was defined as the period from the initial ESD to local or distant recurrence of the index cancer, or death or last patient contact.
Continuous and categorical variables were expressed as mean±standard deviation and n (%), respectively. Patient characteristics were analyzed for the status of NCR by using Student’s t-test for continuous variables and the chi-square test or Fisher’s exact test, as appropriate, for categorical variables. Logistic regression analysis was used to determine the risk factors for NCR. The odds ratio (OR) was considered to be statistically significant if the 95% confidence interval (CI) did not include 1.0. Cumulative local recurrence rate, OS, and DSS were calculated according to the Kaplan–Meier method and analyzed using the log-rank test. Cox proportional hazard regression analysis was used to calculate univariate and multivariate-adjusted hazard ratios and 95% CIs for risk factors for local recurrence in the follow-up. Statistical analysis was performed using SPSS version 25.0 (IBM, Armonk, NY, USA), and the level of statistical significance was set at p<0.05.
Of the 3,094 patients with EGC treated with ESD, 661 patients (21.4%) were found to have undergone NCR. The baseline characteristics of the NCR group are shown in Table 1. No differences in age, family history, smoking history, or comorbidities were seen between the two groups; however, the proportion of male patients in the CR group was higher than that in the NCR group. Certain characteristics of EGC, including undifferentiated carcinoma, upper third location, large tumor, ulceration, and submucosal invasion, were more frequent in the NCR group. With respect to the procedure, the mean procedure time was longer and complications related to the procedure were more frequent in the NCR group. Table 2 shows the characteristics of the NCR group.
Table 3 shows the risk factors for NCR after ESD in patients with EGC. In multivariate regression analysis after adjusting for confounding factors, the risk factors affecting NCR of ESD were old age (OR, 1.42; 95% CI, 1.17–1.74; p<0.001), undifferentiated tumor (OR, 9.47; 95% CI, 7.23–12.41; p<0.001), tumor location in the upper body (OR, 2.17; 95% CI, 1.55–3.05; p<0.001), tumor size ≥2 cm (OR, 3.81; 95% CI, 3.12–4.66; p<0.001), and presence of an ulcer (OR, 1.93; 95% CI, 1.47–2.53; p<0.001).
During the study, the local recurrence rate was significantly higher in the NCR group than in the CR group (10.6% vs. 2.5%, p<0.001) (Table 1). The cumulative local recurrence rate, calculated using Kaplan-Meier analysis, was significantly higher in the NCR group than in the CR group (Fig. 2). However, the cumulative recurrence rate at distant sites was not related to NCR (Fig. 3). Table 4 shows the risk factors affecting local recurrence. In Cox proportional hazard regression analysis, after adjusting for confounding factors, a tumor size of ≥2 cm (OR, 1.51; 95% CI, 1.05–2.17; p<0.028), submucosal invasion (SM1: OR, 1.84; 95% CI, 1.13–3.51; p=0.017 and ≥SM2: OR, 1.84; 95% CI, 1.05–3.21; p=0.033), positive horizontal margin (OR, 3.78; 95% CI, 2.38–6.00; p<0.001), and lymphovascular invasion (OR, 2.58; 95% CI, 1.33–5.01; p=0.005) were risk factors for local recurrence.
A comparison by Kaplan-Meier analysis of the OS of the CR and NCR groups when patients received no additional surgery is shown in Fig. 4. The OS rates at 3, 5, and 10 years were 99.4%, 98.8%, and 97.1%, respectively, in the CR group and 99.4%, 98.9%, and 96.8%, respectively, in the NCR group. There was no statistically significant difference in OS between the two groups (log-rank p=0.788). Fig. 5. shows the Kaplan-Meier analysis of DSS between the two groups. The DSS rates at 3, 5, and 10 years were 100%, 99.9%, and 99.7%, respectively, in the CR group and 99.6%, 99.3%, and 99.3%, respectively, in the NCR group. DSS was significantly lower in the NCR group than in the CR group (log-rank p=0.038).
The aim of this retrospective multicenter study was to analyze the risk factors associated with NCR and the long-term clinical outcomes of NCR with no additional treatment in a large-scale population of patients.
According to several studies, the incidence of NCR after endoscopic resection of EGC is approximately 11.9%–18.5% [15,19,21-23]. In our study, the incidence of NCR was 21.4%, which was somewhat higher than that reported in other studies. In Korea, the number of ESD procedures is steadily increasing because of several factors, including an increase in the number of individual health examinations and the expansion of national cancer screening programs [5]. In addition, ESD is now more frequently performed with larger lesions since the introduction of the expanded criteria for ESD of EGC [13].
The aim of endoscopic resection of EGC is CR; however, unintended NCR may occur owing to various factors. Several previous studies have analyzed the risk factors associated with NCR in endoscopic resection of EGC. In a study of 784 patients who underwent ESD, the risk of NCR was the highest in patients with a tumor size of >3 cm, the presence of an ulcer, and a tumor located in the upper body [21]. In Korea, 1,639 patients with EGC who underwent ESD were retrospectively analyzed for risk factors associated with NCR. The seven factors found to be associated with NCR in that study were large tumor size (≥2 cm), tumor location in the upper body, presence of an ulcer, fusion of gastric folds, absence of mucosal nodularity, spontaneous bleeding, and undifferentiated tumor. The higher the score, the higher the risk of NCR [24]. In addition, a study reported that a tumor size of >2 cm, a superficial elevated and depressed type, and an undifferentiated type were risk factors for NCR [25]. In addition to the characteristics of the lesion at the time of the procedure, the effects of an operator’s endoscopic technique and the pre- and post-procedure discrepancies in NCR were analyzed in one study [22]. By combining the results of these previous studies, the risk factors associated with NCR in endoscopic resection of EGC were large tumor size, tumor location in the upper body, ulceration, undifferentiated tumor, indications falling outside of the absolute and expanded indications for endoscopic resection, improper endoscopic technique, and discrepancies in diagnosis before and after the treatment. These results were not significantly different in our study.
In our study, we analyzed the local recurrence rate and its associated risk factors after ESD. Local recurrence was observed in 10.6% of the ESD sites in the NCR group during the median follow-up period of approximately 50 months, and this rate was significantly higher than the local recurrence rate of 2.5% in the CR group. The risk factors affecting local recurrence in this study were tumor size ≥2 cm, submucosal invasion, positive horizontal margin, and lymphovascular invasion. Previous studies that analyzed the risk factors for local recurrence after endoscopic resection showed similar results. A study that analyzed 152 cases of NCR of EGC reported that there was a high risk of local recurrence in cases of incomplete resection, in cases that exceeded the ESD criteria, and in cases with lymphovascular invasion [26]. A study that analyzed 222 patients with EGC treated with ESD reported that a positive horizontal margin, piecemeal resection, and lymphovascular invasion were risk factors for local recurrence [27]. In two studies in patients with a positive horizontal margin after NCR of EGC, the risk of local recurrence was the highest in patients with a positive horizontal margin of >6 mm length [11,28].
In our study, there was no difference in the OS rate between the CR and NCR groups when patients received no additional treatment, but the DSS rate was significantly lower in the NCR group than in the CR group. This means that the OS rate is offset by the difference between the two groups due to various causes of death; however, given the specificity of survival in gastric cancer, the incidence of gastric cancer-related death in the NCR group was higher than that in the CR group. In other words, additional treatment should be considered for patients with NCR because the DSS rate may be lower without additional treatment in these patients. There are several studies that support our results. In a retrospective study of EGC cases that were outside the indications for endoscopic resection, 1,799 patients who underwent gastrectomy as the first treatment were compared with 219 patients who underwent endoscopic resection as the first treatment, There was no significant difference in mortality and gastric cancer recurrence rates between patients who initially underwent gastrectomy and patients who underwent gastrectomy after NCR; however, the mortality and gastric cancer recurrence rates were significantly higher in patients who did not undergo gastrectomy after NCR than in those who underwent gastrectomy as the first treatment [29].
Our study has some limitations. First, as it is a retrospective study, there may be some bias in patient selection. However, we believe that such a bias is compensated for by the largescale multicenter design. Second, this study included data on local recurrence in the post-ESD prognosis, but no data were collected on lymph node metastasis or distant metastasis after ESD. Third, in the survival analysis, the survival rate was analyzed only in patients with CR and in those who received no additional treatment after NCR. We believe that it is also necessary to analyze the survival rate in patients who received additional treatment, such as redo-ESD or gastrectomy after NCR.
In summary, the risk factors for NCR were older age, undifferentiated tumor, upper location of the tumor, tumor size >2 cm, and presence of an ulcer. The local recurrence rate was significantly higher in NCR, and the risk factors for local recurrence are tumor size >2 cm, submucosal invasion, horizontal margin positive, and lymphovascular invasion. In terms of survival, the DSS rate was significantly lower in NCR without further treatment than in CR. Therefore, if patients can tolerate surgical treatment, additional surgery is recommended in cases of NCR after ESD for EGC.
ACKNOWLEDGMENTS
The authors thank Suck Chei Choi, Jun-Hyung Cho, Kwang Bum Cho, Jae Young Jang, Jae Myung Park, Jae Kyu Sung, Moon Kyung Joo, Jung Won Jeon, Weon Jin Ko, Sun Moon Kim, Yeong Dae Kim, Chan Gyoo Kim, Eun Ran Kim, Gwang Ho Baik, Dong Hoon Baek, Chul-Hyun Lim, and Hyun Joo Jang.
REFERENCES
1. Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014; 23:700–713.

2. Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH, Lee KH. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2014. Cancer Res Treat. 2017; 49:292–305.

3. Tsukuma H, Oshima A, Narahara H, Morii T. Natural history of early gastric cancer: a non-concurrent, long term, follow up study. Gut. 2000; 47:618–621.

4. Mun YG, Choi MG, Lim CH, et al. Factors affecting endoscopic curative resection of gastric cancer in the population-based screening era. Clin Endosc. 2018; 51:478–484.

5. Kang KJ, Lee JH. Characteristics of gastric cancer in Korea - with an emphasis on the increase of the early gastric cancer (EGC). J Korean Med Assoc. 2010; 53:283–289.

6. Kim YI, Kim YW, Choi IJ, et al. Long-term survival after endoscopic resection versus surgery in early gastric cancers. Endoscopy. 2015; 47:293–301.

7. Pyo JH, Lee H, Min BH, et al. Long-term outcome of endoscopic resection vs. surgery for early gastric cancer: a non-inferiority-matched cohort study. Am J Gastroenterol. 2016; 111:240–249.

8. Kim SG, Ji SM, Lee NR, et al. Quality of life after endoscopic submucosal dissection for early gastric cancer: a prospective multicenter cohort study. Gut Liver. 2017; 11:87–92.

9. Kim JH. Strategy for curative endoscopic resection of undifferentiated-type early gastric cancer. Clin Endosc. 2019; 52:9–14.

10. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017; 20:1–19.
11. Sekiguchi M, Suzuki H, Oda I, et al. Risk of recurrent gastric cancer after endoscopic resection with a positive lateral margin. Endoscopy. 2014; 46:273–278.

12. Sekiguchi M, Suzuki H, Oda I, et al. Favorable long-term outcomes of endoscopic submucosal dissection for locally recurrent early gastric cancer after endoscopic resection. Endoscopy. 2013; 45:708–713.

13. Nonaka S, Oda I, Nakaya T, et al. Clinical impact of a strategy involving endoscopic submucosal dissection for early gastric cancer: determining the optimal pathway. Gastric Cancer. 2011; 14:56–62.

14. Bae SY, Jang TH, Min BH, et al. Early additional endoscopic submucosal dissection in patients with positive lateral resection margins after initial endoscopic submucosal dissection for early gastric cancer. Gastrointest Endosc. 2012; 75:432–436.

15. Kim SG. Treatment strategy after incomplete endoscopic resection of early gastric cancer. Clin Endosc. 2016; 49:332–335.

16. Lee SH, Park BS. Is radical surgery necessary for all patients diagnosed as having non-curative endoscopic submucosal dissection? Clin Endosc. 2019; 52:21–29.

17. Ahn JY, Jung HY, Choi JY, et al. Natural course of noncurative endoscopic resection of differentiated early gastric cancer. Endoscopy. 2012; 44:1114–1120.

18. Kawata N, Kakushima N, Takizawa K, et al. Risk factors for lymph node metastasis and long-term outcomes of patients with early gastric cancer after non-curative endoscopic submucosal dissection. Surg Endosc. 2017; 31:1607–1616.

19. Hatta W, Gotoda T, Oyama T, et al. Is radical surgery necessary in all patients who do not meet the curative criteria for endoscopic submucosal dissection in early gastric cancer? A multi-center retrospective study in Japan. J Gastroenterol. 2017; 52:175–184.

20. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011; 14:101–112.

21. Hirasawa K, Kokawa A, Oka H, et al. Risk assessment chart for curability of early gastric cancer with endoscopic submucosal dissection. Gastrointest Endosc. 2011; 74:1268–1275.

22. Toyokawa T, Inaba T, Omote S, et al. Risk factors for non-curative resection of early gastric neoplasms with endoscopic submucosal dissection: analysis of 1,123 lesions. Exp Ther Med. 2015; 9:1209–1214.

23. Suzuki H, Oda I, Abe S, et al. Clinical outcomes of early gastric cancer patients after noncurative endoscopic submucosal dissection in a large consecutive patient series. Gastric Cancer. 2017; 20:679–689.

24. Kim EH, Park JC, Song IJ, et al. Prediction model for non-curative resection of endoscopic submucosal dissection in patients with early gastric cancer. Gastrointest Endosc. 2017; 85:976–983.

25. Ohara Y, Toshikuni N, Matsueda K, Mouri H, Yamamoto H. The superficial elevated and depressed lesion type is an independent factor associated with non-curative endoscopic submucosal dissection for early gastric cancer. Surg Endosc. 2016; 30:4880–4888.

26. Han JP, Hong SJ, Kim HK, et al. Risk stratification and management of non-curative resection after endoscopic submucosal dissection for early gastric cancer. Surg Endosc. 2016; 30:184–189.

27. Kim JH, Lee JH, Chung JW, et al. Risk factors for local recurrence of early gastric cancer after endoscopic submucosal dissection. Korean J Med. 2013; 85:285–293.

Fig. 1.
Flowchart of patient enrollment. EGCA, early gastric cancer; EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; Hx, history; NCR, non-curative resection.
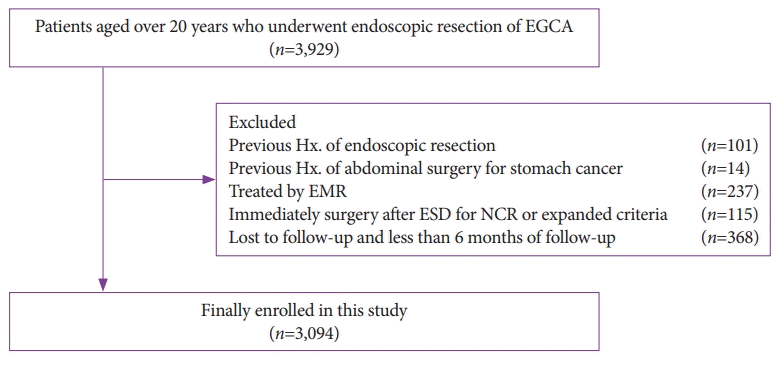
Fig. 2.
Kaplan-Meier analysis for cumulative recurrence rates at previous endoscopic submucosal dissection site according to non-curative resection. Log-rank p<0.001.
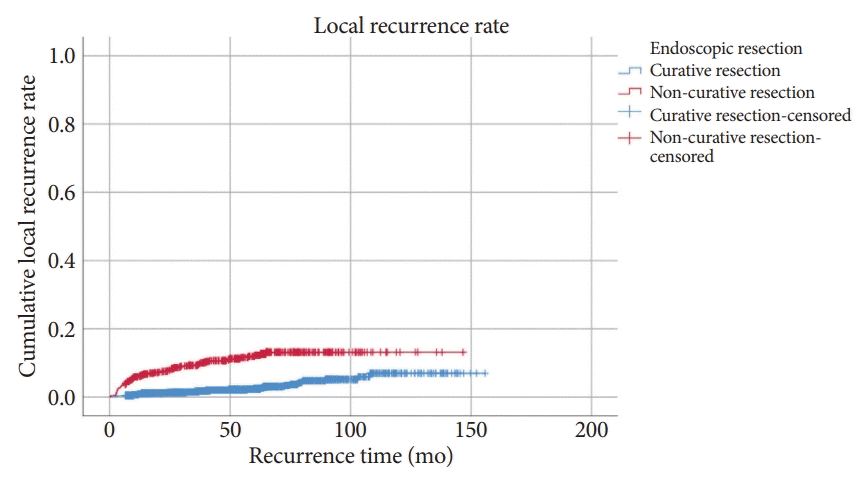
Fig. 3.
Kaplan-Meier analysis for cumulative recurrence rates at other site according to non-curative resection. Log-rank p=0.585.
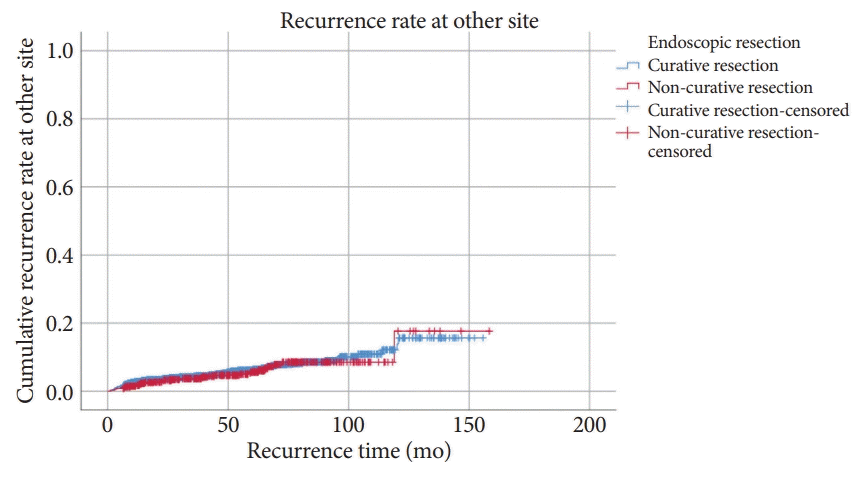
Fig. 4.
Kaplan-Meier analysis for cumulative overall survival rates according to non-curative resection. Log-rank p=0.788.
Fig. 5.
Kaplan-Meier analysis for cumulative disease-specific survival rates according to non-curative resection. Logrank p=0.038.
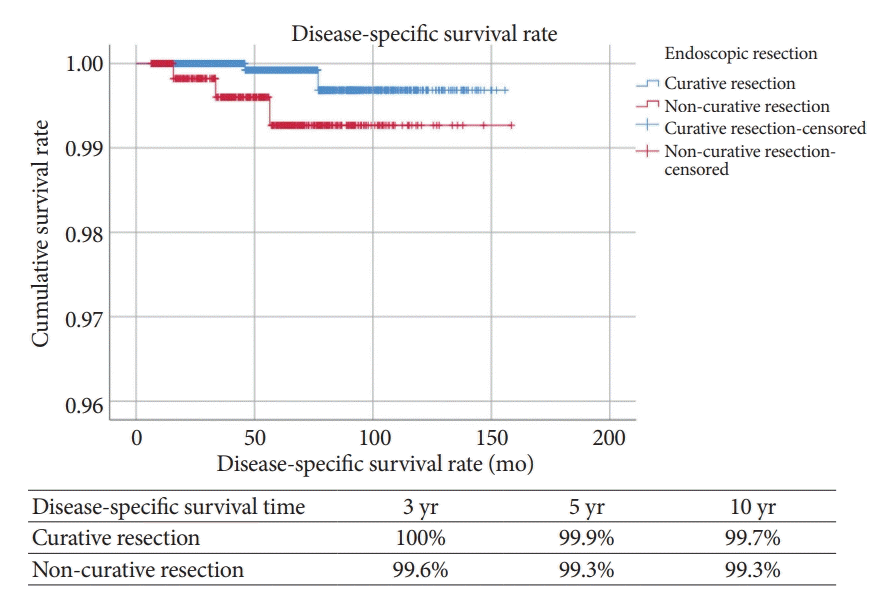
Table 1.
Baseline Characteristics
Table 2.
Characteristics of Non-Curative Resection
Table 3.
Risk Factors for Non-Curative Resection
Table 4.
Risk Factors Affecting Local Recurrence at Previous Endoscopic Submucosal Dissection Site after Endoscopic Submucosal Dissection
| Variables |
Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age | ||||
| <65 yr | 1 | 0.342 | ||
| ≥65 yr | 1.19 (0.84–1.68) | |||
| Sex | ||||
| Male | 1 | 0.067 | 1 | 0.105 |
| Female | 0.67 (0.44–1.03) | 0.71 (0.47–1.08) | ||
| Post-procedure diagnosis | ||||
| Differentiated | 1 | 0.136 | ||
| Undifferentiated | 1.45 (0.89–2.37) | |||
| Tumor location | ||||
| Lower | 1 | |||
| Middle | 1.15 (0.78–1.69) | 0.482 | ||
| Upper | 1.25 (0.69–2.26) | 0.465 | ||
| Tumor size | ||||
| <2 cm | 1 | 0.071 | 1 | 0.028 |
| ≥2 cm | 1.41 (0.97–2.04) | 1.51 (1.05–2.17) | ||
| Depth of tumor | ||||
| M | 1 | 1 | ||
| SM1 | 1.82 (1.01–3.27) | 0.046 | 1.99 (1.13–3.51) | 0.017 |
| ≥SM2 | 1.59 (0.86–2.93) | 0.137 | 1.84 (1.05–3.21) | 0.033 |
| Horizontal margin positive | ||||
| No | 1 | <0.001 | 1 | <0.001 |
| Yes | 3.50 (2.17–5.64) | 3.78 (2.38–6.00) | ||
| Vertical margin positive | ||||
| No | 1 | 0.608 | ||
| Yes | 1.22 (0.57–2.60) | |||
| Lymphovascular invasion | ||||
| No | 1 | 0.005 | 1 | 0.005 |
| Yes | 2.65 (1.35–5.21) | 2.58 (1.33–5.01) | ||
| Helicobacter pylori infection state | ||||
| Noa) | 1 | |||
| Yesb) | 1.25 (0.80–1.93) | 0.331 | ||
| Un-evaluated | 1.17 (0.74–1.84) | 0.510 | ||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download