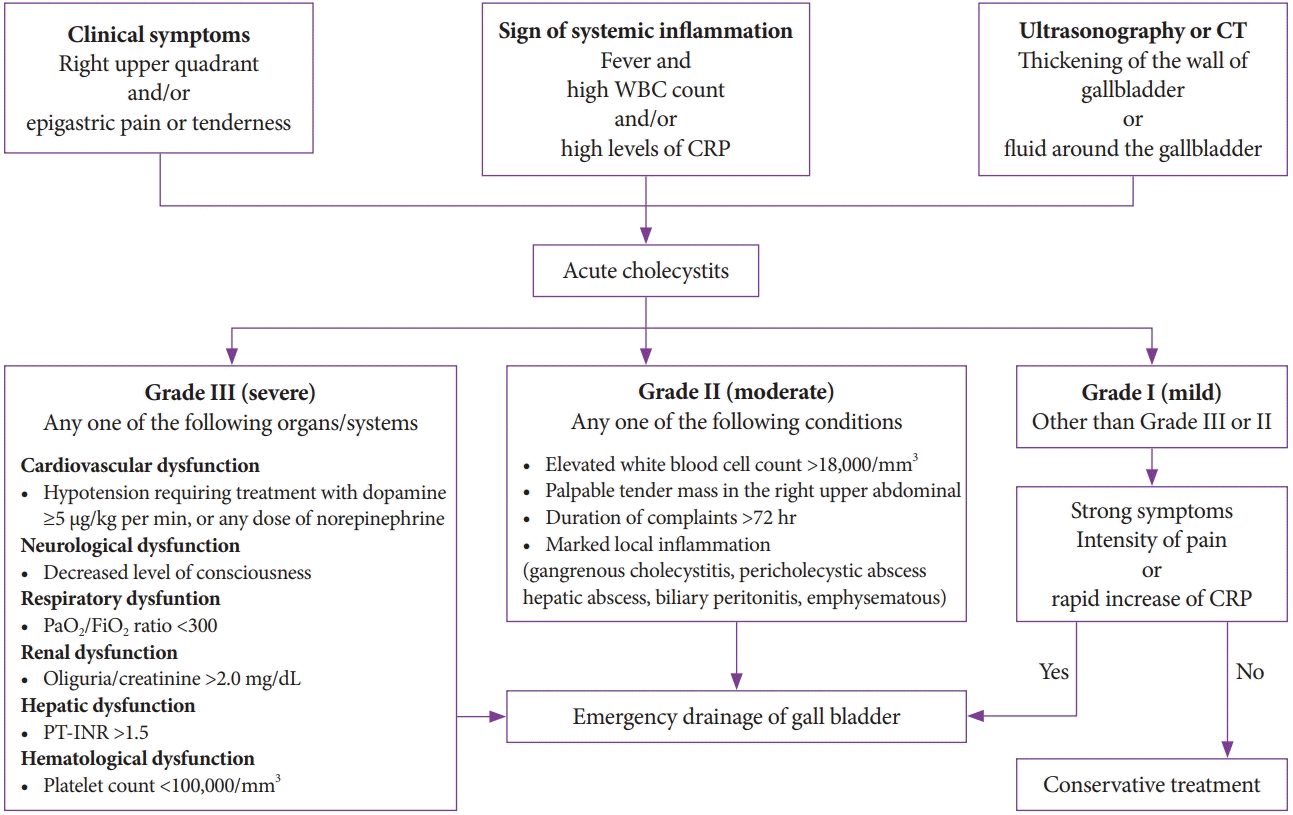
1. Yokoe M, Hata J, Takada T, et al. Tokyo guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018; 25:41–54.
2. Hannan EL, Imperato PJ, Nenner RP, Starr H. Laparoscopic and open cholecystectomy in New York state: mortality, complications, and choice of procedure. Surgery. 1999; 125:223–231.

3. Duncan CB, Riall TS. Evidence-based current surgical practice: calculous gallbladder disease. J Gastrointest Surg. 2012; 16:2011–2025.

4. Csikesz NG, Tseng JF, Shah SA. Trends in surgical management for acute cholecystitis. Surgery. 2008; 144:283–289.

5. Margiotta SJ Jr, Horwitz JR, Willis IH, Wallack MK. Cholecystectomy in the elderly. Am J Surg. 1988; 156:509–512.

6. Glenn F. Cholecystostomy in the high risk patient with biliary tract disease. Ann Surg. 1977; 185:185–191.

7. Roslyn JJ, Binns GS, Hughes EF, Saunders-Kirkwood K, Zinner MJ, Cates JA. Open cholecystectomy. A contemporary analysis of 42,474 patients. Ann Surg. 1993; 218:129–137.

8. Chopra S, Dodd GD 3rd, Mumbower AL, et al. Treatment of acute cholecystitis in non-critically ill patients at high surgical risk: comparison of clinical outcomes after gallbladder aspiration and after percutaneous cholecystostomy. AJR Am J Roentgenol. 2001; 176:1025–1031.
9. Ito K, Fujita N, Noda Y, et al. Percutaneous cholecystostomy versus gallbladder aspiration for acute cholecystitis: a prospective randomized controlled trial. AJR Am J Roentgenol. 2004; 183:193–196.

10. Sosna J, Copel L, Kane RA, Kruskal JB. Ultrasound-guided percutaneous cholecystostomy: update on technique and clinical applications. Surg Technol Int. 2003; 11:135–139.
11. Patel M, Miedema BW, James MA, Marshall JB. Percutaneous cholecystostomy is an effective treatment for high-risk patients with acute cholecystitis. Am Surg. 2000; 66:33–37.
12. Griniatsos J, Petrou A, Pappas P, et al. Percutaneous cholecystostomy without interval cholecystectomy as definitive treatment of acute cholecystitis in elderly and critically ill patients. South Med J. 2008; 101:586–590.

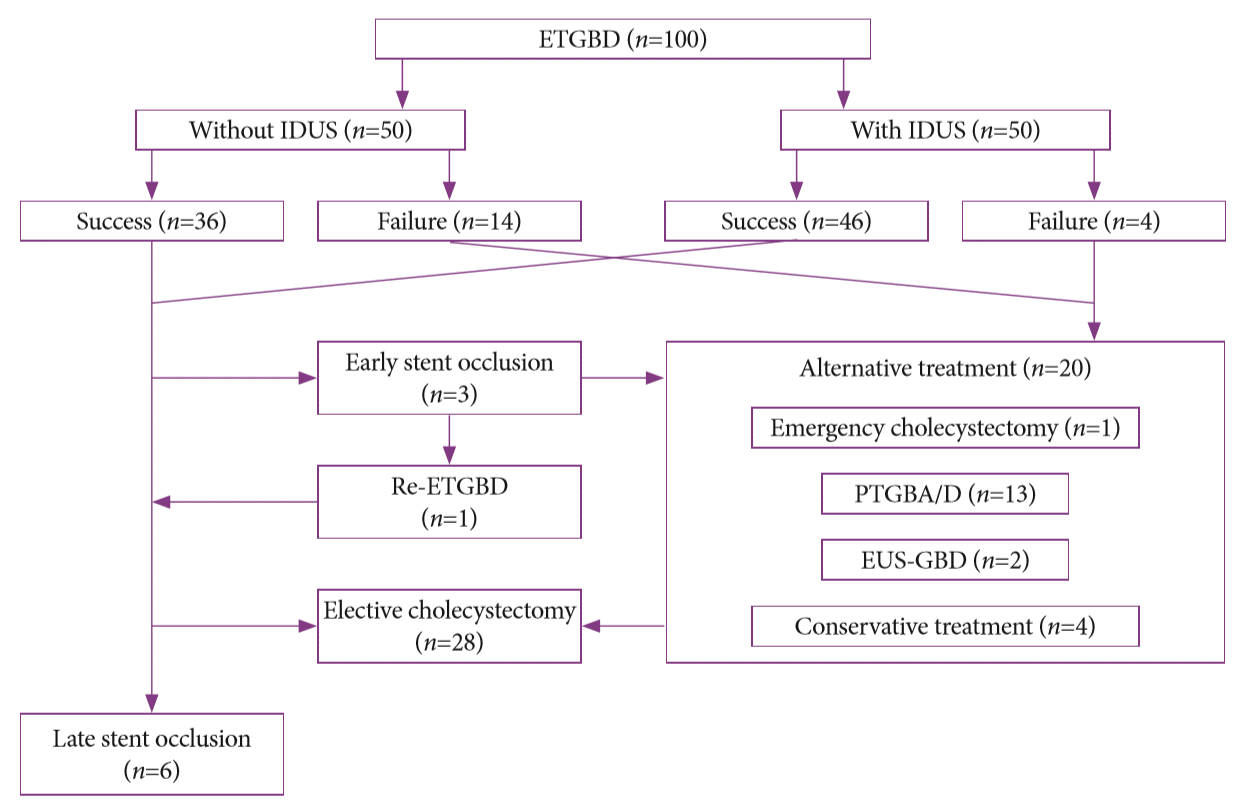
13. Itoi T, Sofuni A, Itokawa F, et al. Endoscopic transpapillary gallbladder drainage in patients with acute cholecystitis in whom percutaneous transhepatic approach is contraindicated or anatomically impossible (with video). Gastrointest Endosc. 2008; 68:455–460.

14. Hasan MK, Itoi T, Varadarajulu S. Endoscopic management of acute cholecystitis. Gastrointest Endosc Clin N Am. 2013; 23:453–459.

15. Mori Y, Itoi T, Baron TH, et al. Tokyo guidelines 2018: management strategies for gallbladder drainage in patients with acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018; 25:87–95.
16. Burke DR, Lewis CA, Cardella JF, et al. Quality improvement guidelines for percutaneous transhepatic cholangiography and biliary drainage. J Vasc Interv Radiol. 2003; 14(9 Pt 2):S243–S246.

17. Hatzidakis AA, Prassopoulos P, Petinarakis I, et al. Acute cholecystitis in high-risk patients: percutaneous cholecystostomy vs conservative treatment. Eur Radiol. 2002; 12:1778–1784.

18. Ha JP, Tsui KK, Tang CN, Siu WT, Fung KH, Li MK. Cholecystectomy or not after percutaneous cholecystostomy for acute calculous cholecystitis in high-risk patients. Hepatogastroenterology. 2008; 55:1497–1502.
19. Tamada K, Seki H, Sato K, et al. Efficacy of endoscopic retrograde cholecystoendoprosthesis (ERCCE) for cholecystitis. Endoscopy. 1991; 23:2–3.

20. Feretis C, Apostolidis N, Mallas E, Manouras A, Papadimitriou J. Endoscopic drainage of acute obstructive cholecystitis in patients with increased operative risk. Endoscopy. 1993; 25:392–395.

21. Kalloo AN, Thuluvath PJ, Pasricha PJ. Treatment of high-risk patients with symptomatic cholelithiasis by endoscopic gallbladder stenting. Gastrointest Endosc. 1994; 40:608–610.

22. Gaglio PJ, Buniak B, Leevy CB. Primary endoscopic retrograde cholecystoendoprosthesis: a nonsurgical modality for symptomatic cholelithiasis in cirrhotic patients. Gastrointest Endosc. 1996; 44:339–342.

23. Itoi T, Coelho-Prabhu N, Baron TH. Endoscopic gallbladder drainage for management of acute cholecystitis. Gastrointest Endosc. 2010; 71:1038–1045.

24. Yu W, Li W, Wang Z, Ye X, Li N, Li J. Early percutaneous transhepatic gallbladder drainage compared with endoscopic retrograde cholangiopancreatography and papillotomy treatment for severe gallstone associated acute pancreatitis. Postgrad Med J. 2007; 83:187–191.

25. Widmer J, Alvarez P, Sharaiha RZ, et al. Endoscopic gallbladder drainage for acute cholecystitis. Clin Endosc. 2015; 48:411–420.

26. Súbtil JC, Betes M, Muñoz-Navas M. Gallbladder drainage guided by endoscopic ultrasound. World J Gastrointest Endosc. 2010; 2:203–209.

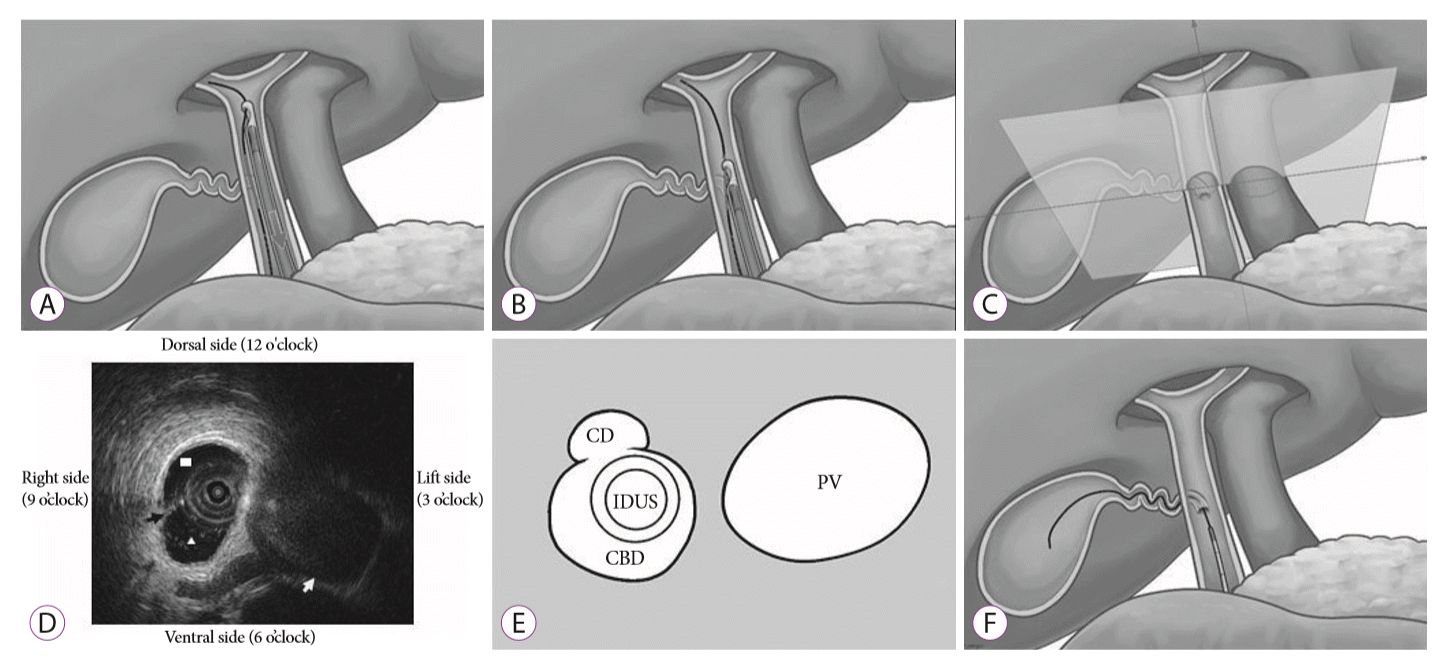
27. Hayasaka K, Harada H, Shimizu T, Suehiro S, Katsuyama Y. The effectiveness of intraductal ultrasonography for cystic duct cannulation. Gastrointest Endosc. 2017; 85:1307–1308.
28. Ogawa O, Yoshikumi H, Maruoka N, et al. Predicting the success of endoscopic transpapillary gallbladder drainage for patients with acute cholecystitis during pretreatment evaluation. Can J Gastroenterol. 2008; 22:681–685.

29. Kjaer DW, Kruse A, Funch-Jensen P. Endoscopic gallbladder drainage of patients with acute cholecystitis. Endoscopy. 2007; 39:304–308.

30. Pannala R, Petersen BT, Gostout CJ, Topazian MD, Levy MJ, Baron TH. Endoscopic transpapillary gallbladder drainage: 10-year single center experience. Minerva Gastroenterol Dietol. 2008; 54:107–113.
31. Mutignani M, Iacopini F, Perri V, et al. Endoscopic gallbladder drainage for acute cholecystitis: technical and clinical results. Endoscopy. 2009; 41:539–546.

32. Khan MA, Atiq O, Kubiliun N, et al. Efficacy and safety of endoscopic gallbladder drainage in acute cholecystitis: is it better than percutaneous gallbladder drainage? Gastrointest Endosc. 2017; 85:76–87.e3.

33. McCarthy ST, Tujios S, Fontana RJ, et al. Endoscopic transpapillary gallbladder stent placement is safe and effective in high-risk patients without cirrhosis. Dig Dis Sci. 2015; 60:2516–2522.

34. Barkay O, Bucksot L, Sherman S. Endoscopic transpapillary gallbladder drainage with the SpyGlass cholangiopancreatoscopy system. Gastrointest Endosc. 2009; 70:1039–1040.

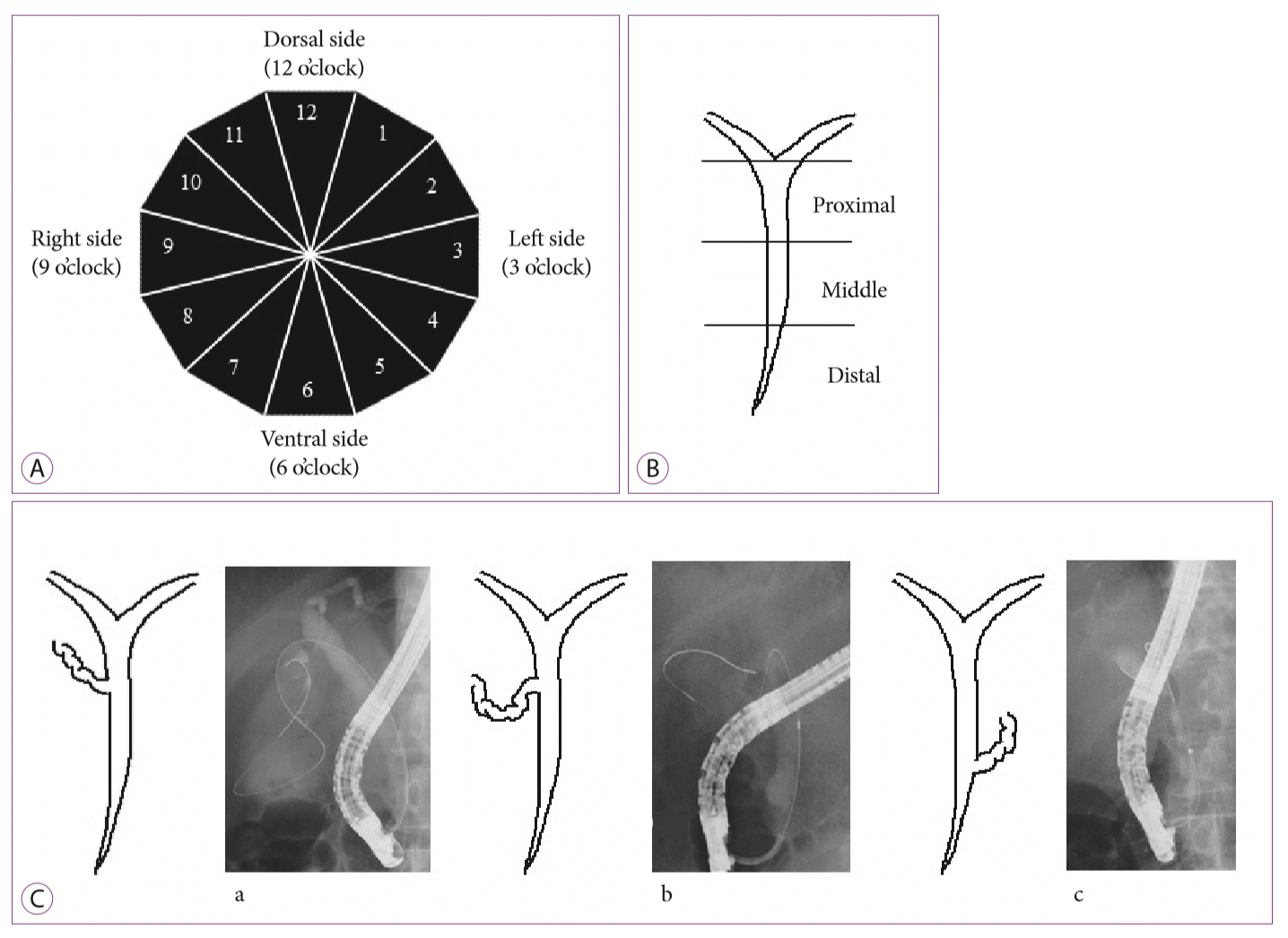
35. Shaw MJ, Dorsher PJ, Vennes JA. Cystic duct anatomy: an endoscopic perspective. Am J Gastroenterol. 1993; 88:2102–2106.
36. Berci G. Biliary ductal anatomy and anomalies. The role of intraoperative cholangiography during laparoscopic cholecystectomy. Surg Clin North Am. 1992; 72:1069–1075.
37. Itoi T, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Niito T. Endoscopic transpapillary gallbladder drainage. Gastroenterological Endoscopy. 2010; 52:3337–3346.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download