Abstract
Background/Aims:
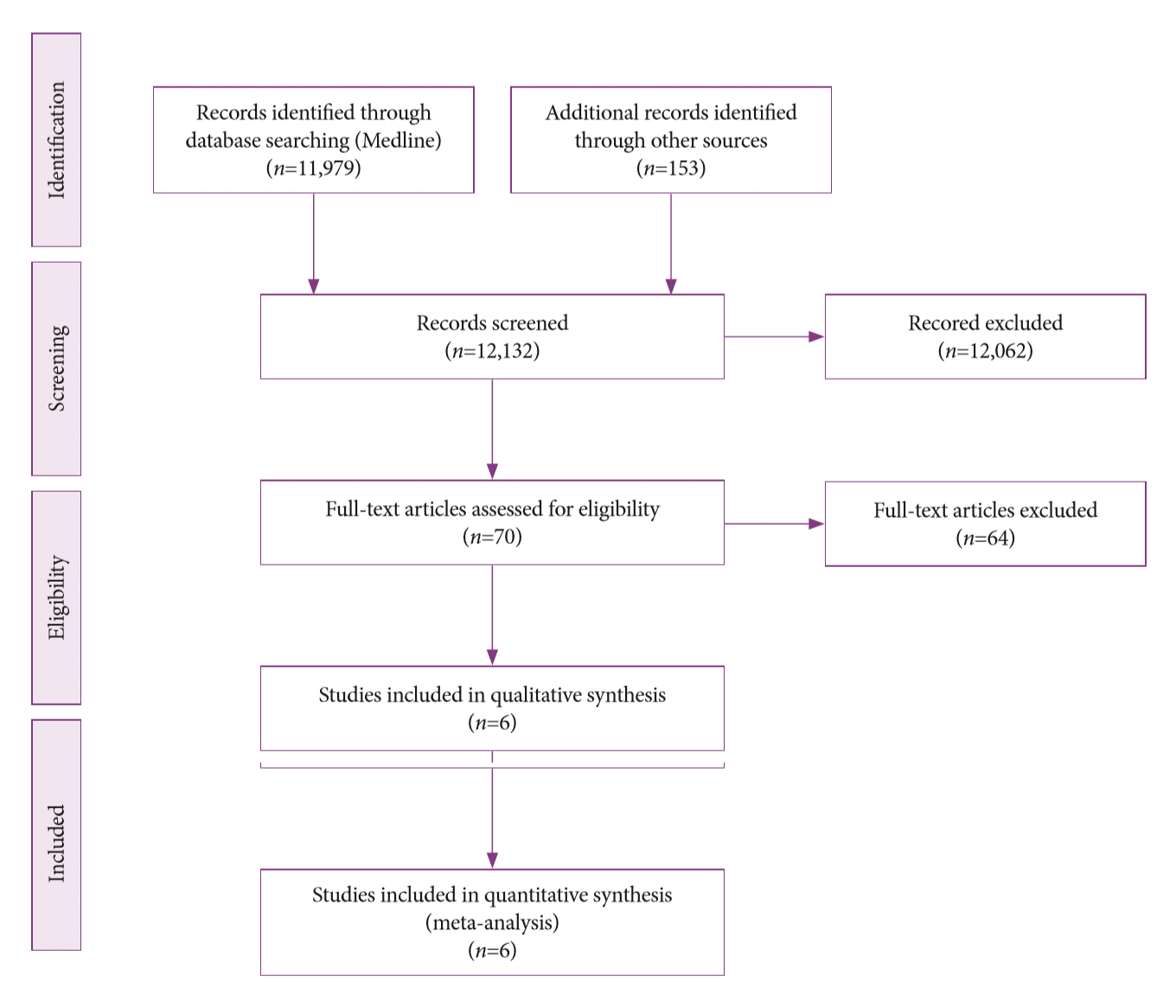
Methods:
Results:
Notes
Conflicts of Interest: Marvin Ryou is a consultant for Olympus and Medtronic, Eduardo Guimarães Hourneaux de Moura is a consultant for Boston Scientific and Olympus, Christopher C. Thompson is consultant for Boston Scientific, Olympus and Medtronic. The other authors have no financial conflicts of interest.
Author Contributions
Conceptualization: Diogo Turiani Hourneax de Moura
Data curation: DTHM, Igor Braga Ribeiro
Formal analysis: Wanderlei Marques Bernardo
Methodology: DTHM, WMB, Eduardo Guimarães Hourneaux de Moura
Project administration: EGHM, WMB
Supervision: EGHM, Christopher C. Thompson
Writing-original draft: DTHM
Writing-review&editing: Marvin Ryou, CCT
REFERENCES
Fig. 2.
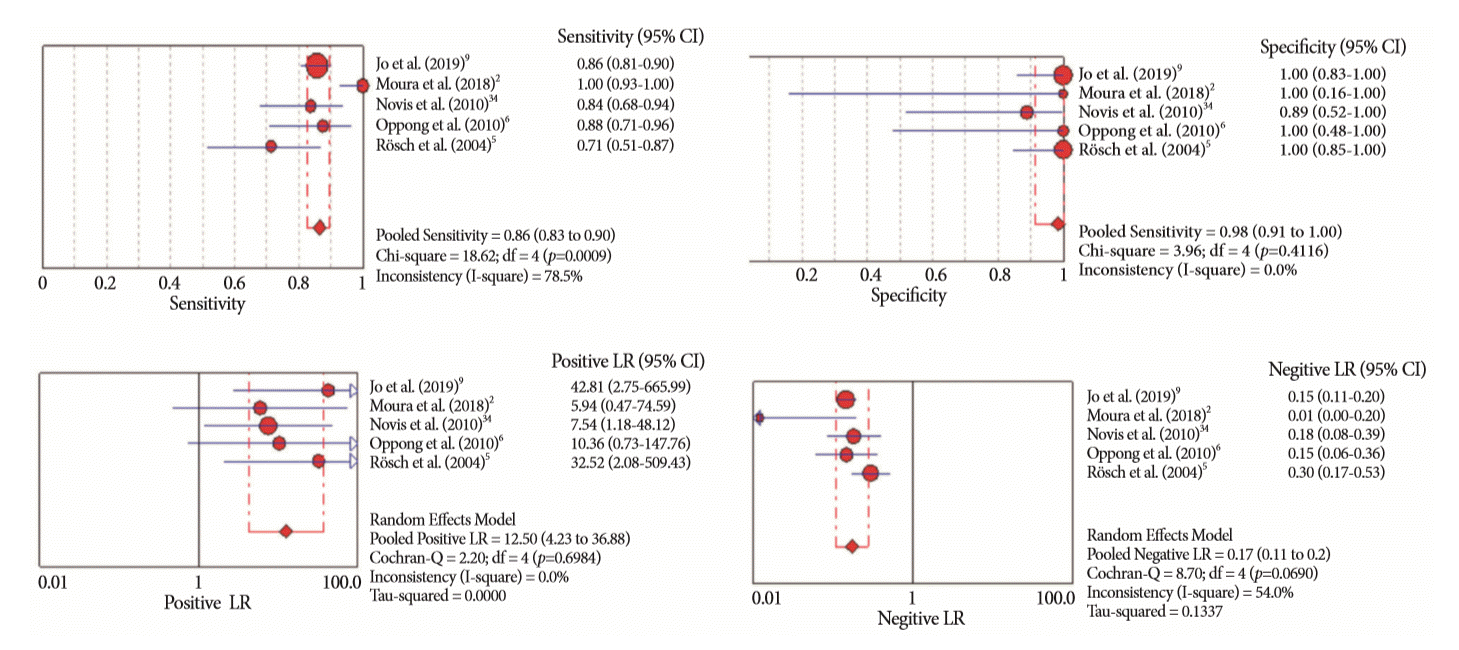
Fig. 3.
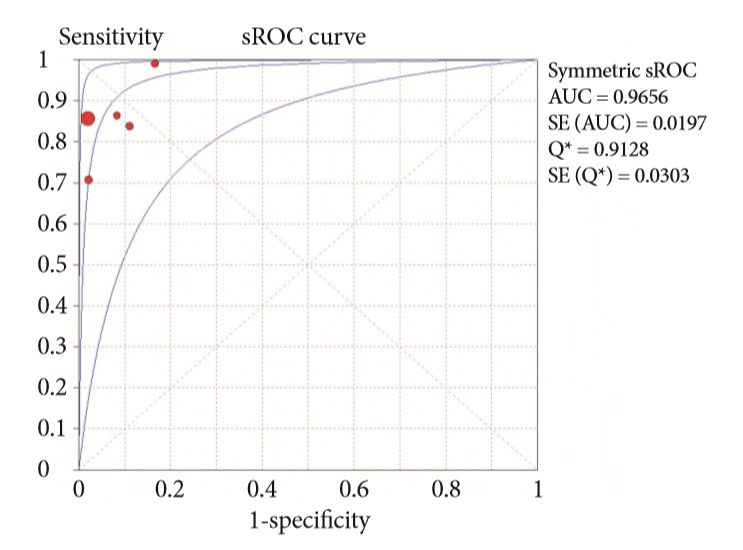
Fig. 4.
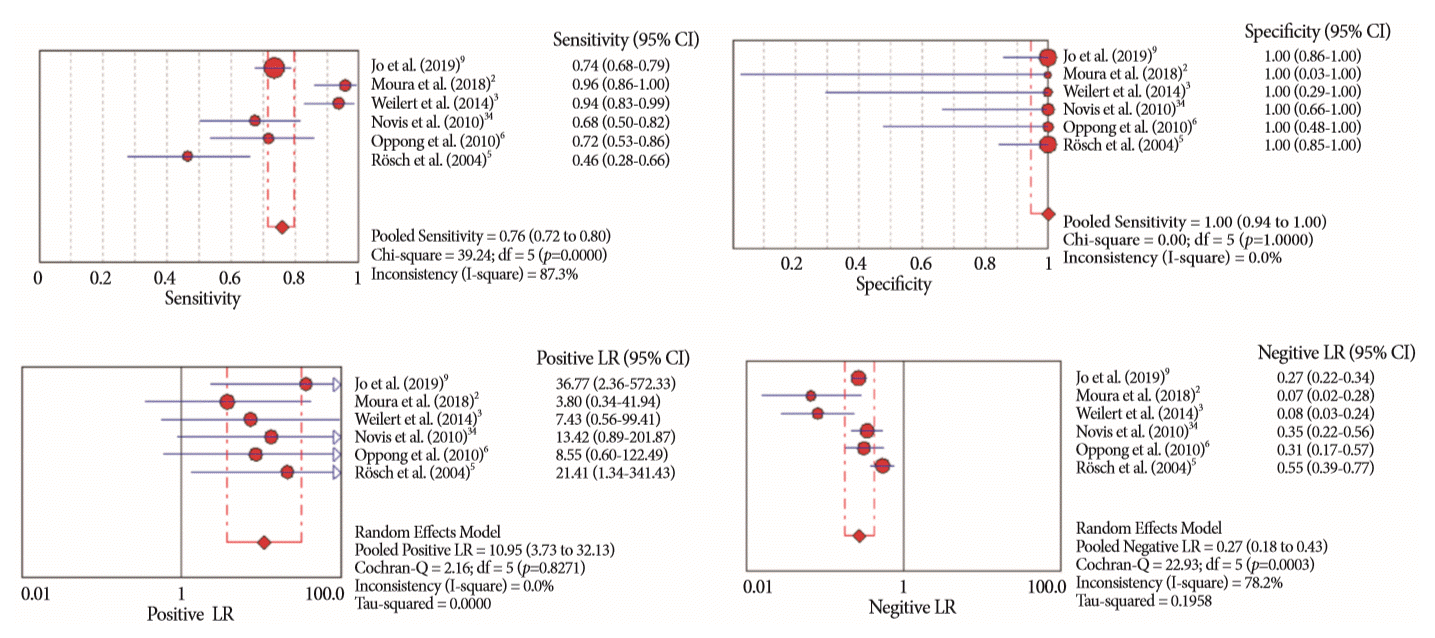
Fig. 5.
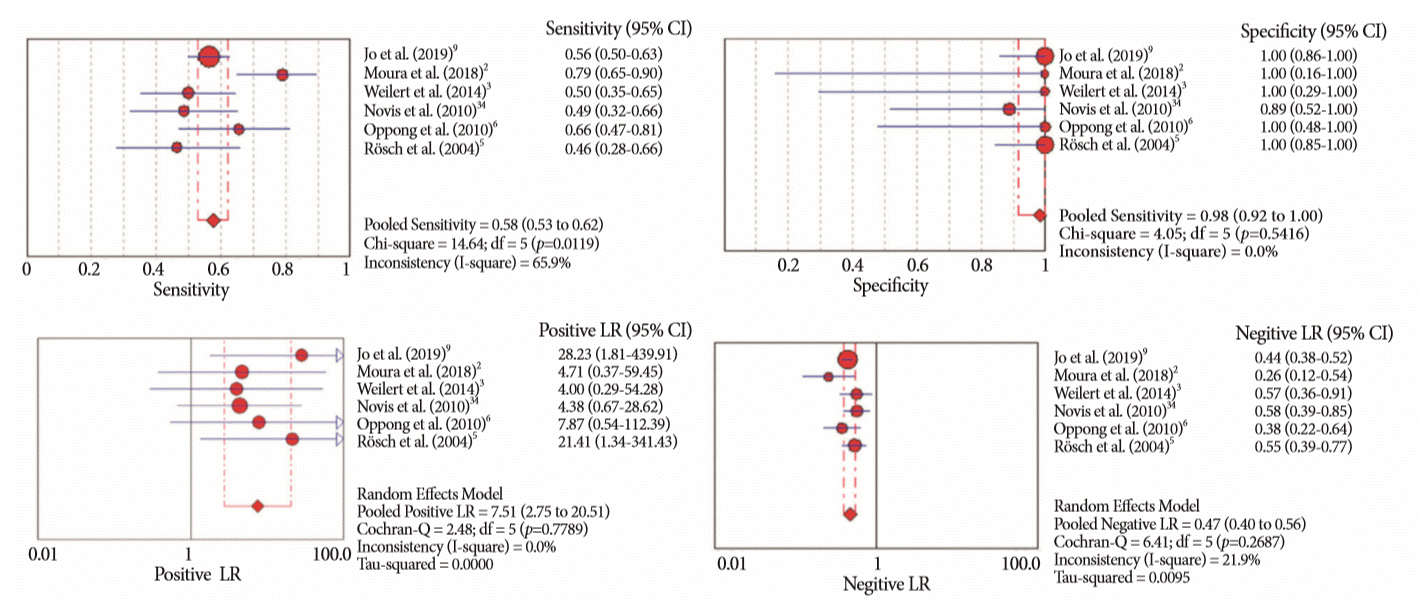
Fig. 6.
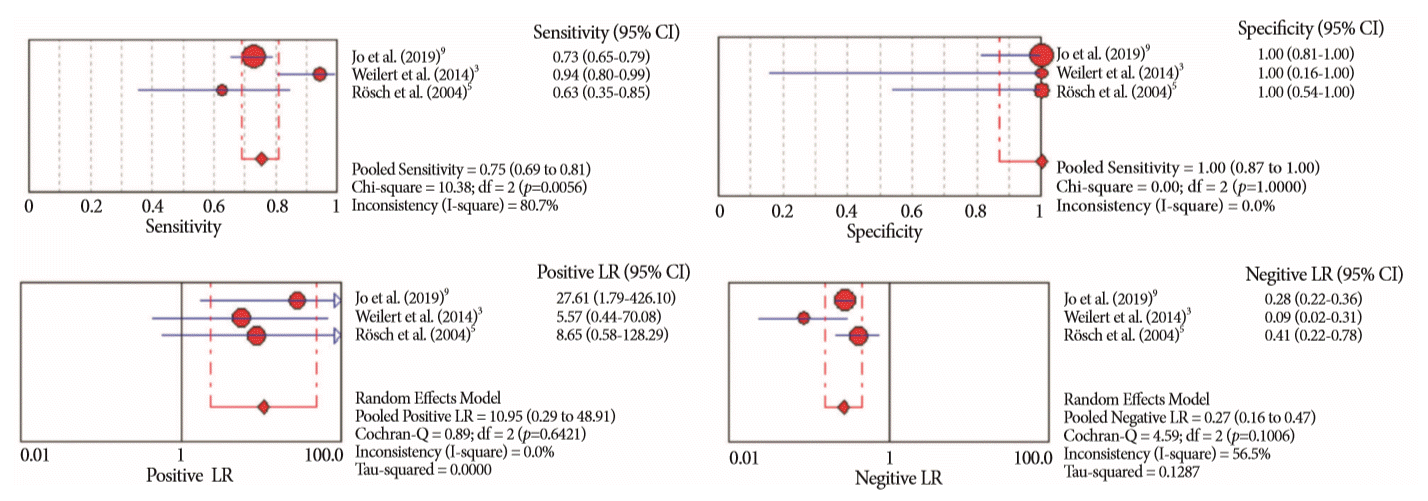
Fig. 7.
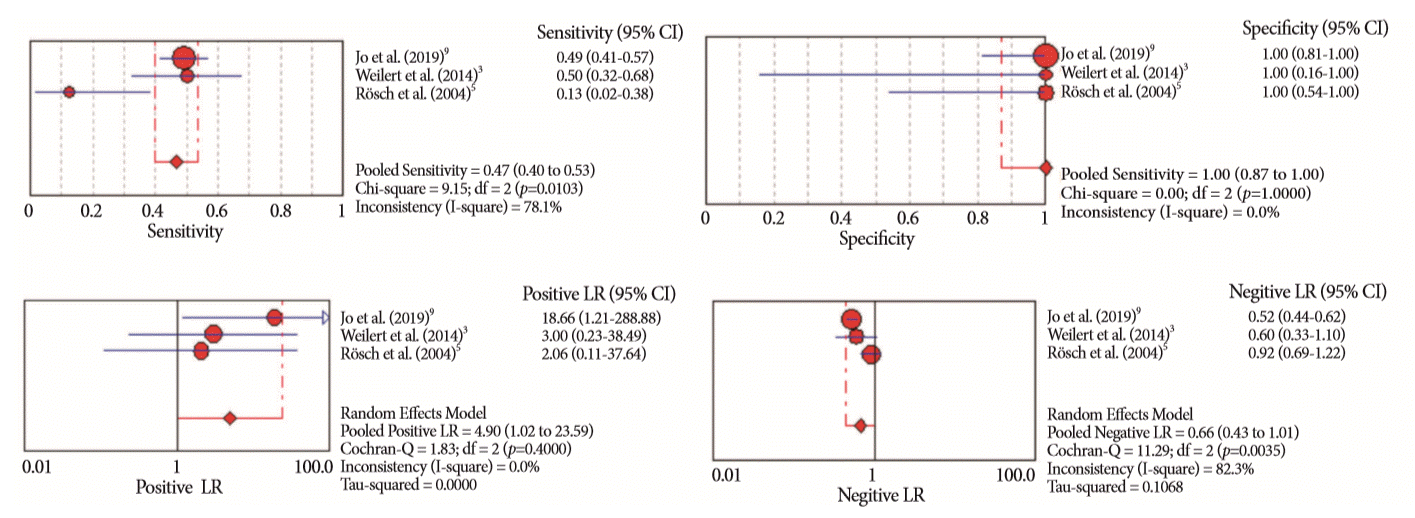
Fig. 8.
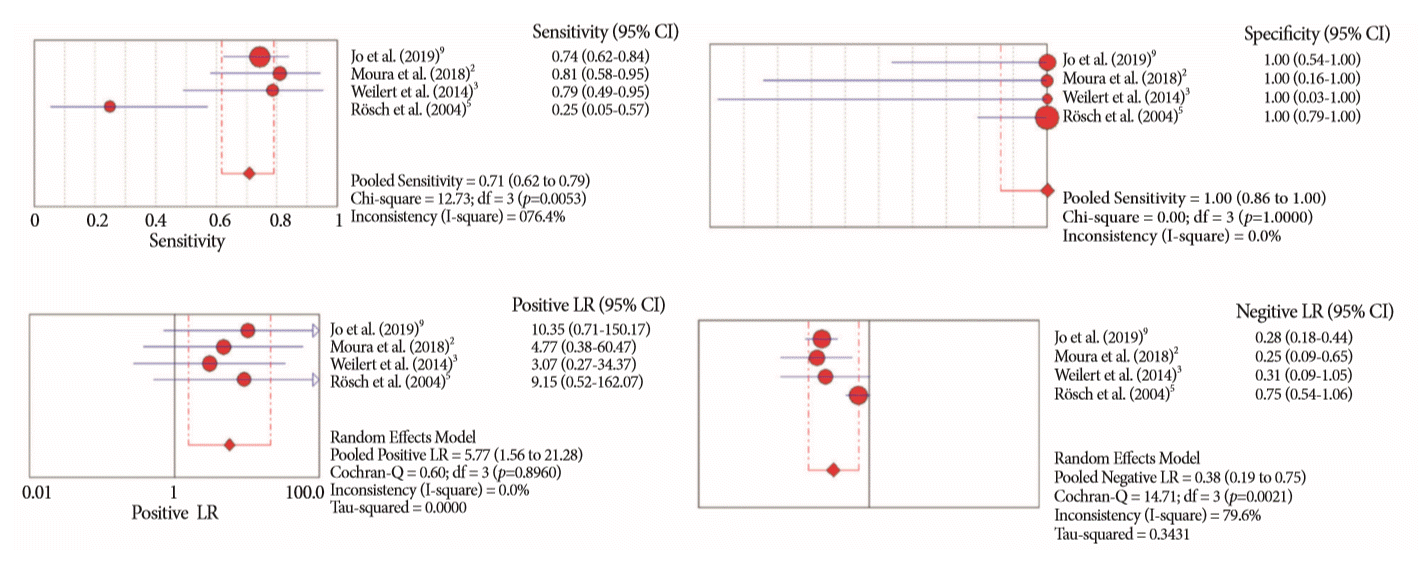
Fig. 9.
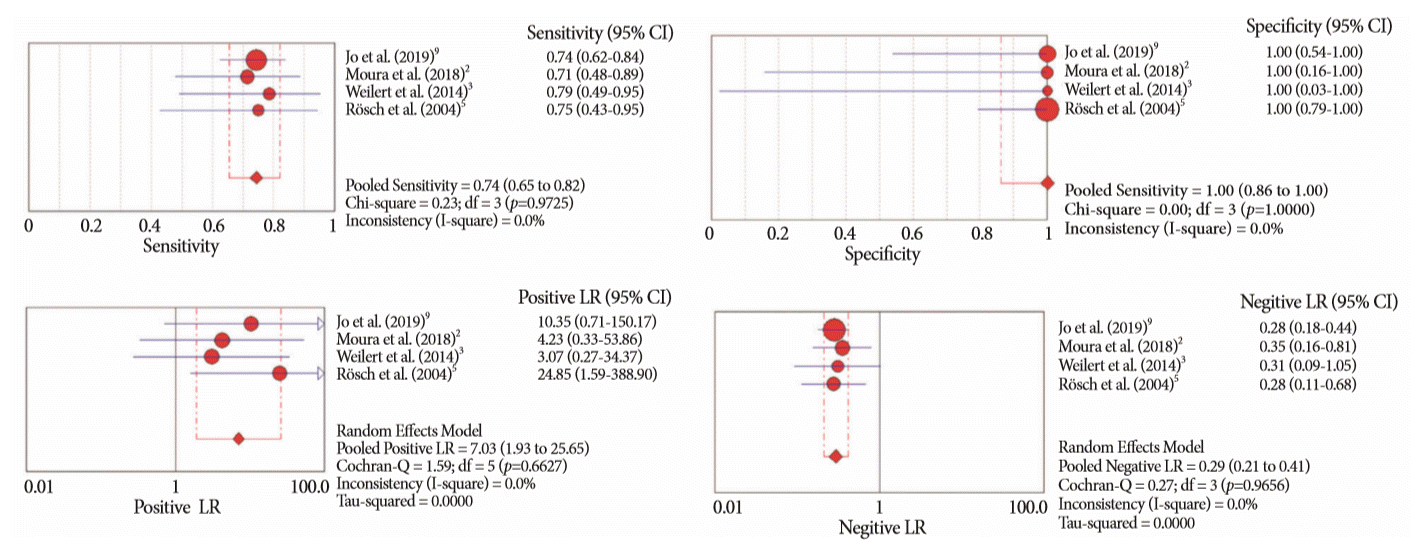
Table 1.
| Study | Patients (n) | Age (yr) | Lesion size | Intervention | Gold standard | Final diagnosis |
|---|---|---|---|---|---|---|
| Jo et al. (2019) [9] | 263 | 64.6±10.5 | 26.9±11.6 mm | EUS-FNA (22 G, 25H, 20 G and 19 G): 2.7 (±1.2) passes | 1) Surgical pathology; | Malignant: 239 |
| M: 167 | 2) pathologic diagno- sis made by any tissue acquisition method; 3) follow-up (>6 mo) | - Pancreatic mass: 163 | ||||
| Design: Retrospective | F: 96 | ERCP: 3 (1–7) intraductal biopsy in 246/257 cases and cytology (via endoscopic nasobiliary drainage or brushing in all cases) | - CCA: 53 | |||
| - Gallbladder cancer: 14 | ||||||
| - Other: 9 | ||||||
| Benign: 24 | ||||||
| - Autoimmune pancreatitis: 12 | ||||||
| - Chronic pancreatitis: 5 | ||||||
| - Other: 7 | ||||||
| Moura et al. (2018) [2] | 50 | 63.08 (41–86) | 3.48±1.72 cm | EUS-FNA (22 G): 4 passes | 1) Surgical pathology; | Malignant: 48 |
| M: 24 | 2) clinical follow-up (>6 mo) | - Adenocarcinoma: 36 | ||||
| Design: Prospective | F: 26 | ERCP: 3 intraductal biopsies and 2 brush cytology | - IPMN: 4 | |||
| - Metastases: 3 | ||||||
| - Neuroendocrine tumor: 2 | ||||||
| - Adenosquamous: 1 | ||||||
| Weilert et al. (2014) [3] | 51 | 67 (42–88) | N/A | EUS-FNA (22 G or 25 G)—with ROSE | 1) Surgical findings/pathology; 2) EUS or ERCP sampling with definite evidence for malignancy; and 3) clinical follow-up (>6 mo) | Malignant: 48 |
| - Pancreatic cancer: 34 | ||||||
| Design: Prospective | ERCP: 2 to 3 intraductal biopsies and brush cytology | - CCA: 13 | ||||
| - Gallbladder cancer: 1 | ||||||
| Benign: 3 | ||||||
| - Autoimmune pancreatitis: 1 | ||||||
| - Chronic pancreatitis: 1 | ||||||
| - Autoimmune cholangiopathy: 1 | ||||||
| Novis et al. (2010) [34] | 46 | 56 (40–87) | N/A | EUS-FNA (22 G): at least 3 passes-with ROSE (by the endoscopist) | 1) Surgical pathology; 2) EUS or ERCP sampling with evidence for malignancy; and 3) clinical follow-up (>6 mo for malignance and 24 mo for benign) | Malignant: 37 |
| M: 21 | - Pancreatic cancer: 26 | |||||
| Design: Prospective | F: 25 | ERCP brush cytology | - Biliary: 11 | |||
| - Common bile duct: 8 | ||||||
| - Hilar tumors: 3 | ||||||
| Benign: 9 | ||||||
| - Chronic pancreatitis: 8 | ||||||
| - Fibrosis: 2 | ||||||
| Oppong et al. (2010) [6] | 37 | 62.4 (26–87) | N/A | EUS-FNA (22 G and 25 G): 2.7 (1–6) passes | 1) Surgical histology or other biopsy methods; | Malignant: 32 |
| - Pancreatic tumor: 29 | ||||||
| Design: Retrospective | ERCP brush cytology: at least 3 brushings | 2) any positive cytology result combined with clinical follow-up with evidence of malignancy; 3) follow-up until death or for at least two years if there was no evidence of malignancy | - Neuroendocrine tumor: 2 | |||
| - CCA: 1 | ||||||
| Benign: 5 | ||||||
| - Chronic pancreatitis: 2 | ||||||
| - Primary sclerosing cholangitis: 1 | ||||||
| - Serous cyst adenoma: 1 | ||||||
| - GIST: 1 | ||||||
| Rösch et al. (2004) [5] | 50 | N/A | N/A | EUS-FNA (22 G): at least 2 passes | 1) Surgery pathology | Malignant: 28 |
| M: 29 | ERCP: 6 intraductal biopsies and brush cytology (2 types of brush, 2 passes with each) | 2) Biopsy specimens obtained by other methods | - Pancreatic tumors: 16 | |||
| Design: Prospective | F: 21 | 3) A positive result for any tissue acquisition method being evaluated, plus clinical follow-up that provided further evidence of malignancy | - Biliary tumors: 12 (8 common bile duct and 4 hilar) | |||
| 4) Further evidence of malignancy (e.g., distant metastases) | Benign: 22 | |||||
| - Chronic pancreatitis 6 | ||||||
| 5) 6-mo follow-up | - CBD stricture: 16 (9 common bile duct and 7 hilar) |
Table 2.
| Study |
Risk of bias |
Applicability concerns |
|||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Jo et al. (2019) [9] |

|

|

|

|

|

|

|
| Moura et al. (2018) [2] |

|

|

|

|

|

|

|
| Weilert et al. (2014) [3] |

|

|

|

|

|

|

|
| Novis et al. (2010) [34] |

|

|

|

|

|

|

|
| Oppong et al. (2010) [6] |

|

|

|

|

|

|

|
| Rösch et al. (2004) [5] |

|

|

|

|

|

|

|
Table 3.
Table 4.
| Study | Patients, n | Adverse events, n(%) | Adverse events |
|---|---|---|---|
| Jo et al. (2019) [9] | 263 | 24 (9.12) | - 8 bleedings |
| - 2 cholangitis | |||
| - 14 pancreatitis | |||
| Moura et al. (2018) [2] | 50 | 3 (6) | - 2 mild pancreatitis |
| - 1 post sphincterotomy bleeding without hemodynamic repercussion, treated endoscopically | |||
| Weilert et al. (2014) [3] | 51 | 0 | No adverse events |
| Novis et al. (2010) [34] | 46 | 5 (10.86) | - 2 cholangitis treated endoscopically |
| - 1 mild pancreatitis | |||
| - 1 biliary peritonitis. Surgical intervention was required. Patient died after surgery | |||
| - 1 mild bleeding. No intervention was required | |||
| Oppong et al. (2010) [6] | 37 | 2 (9.1) | - 1 mild pancreatitis |
| - 1 inadequate biliary drainage after procedures. Stent exchange was required | |||
| Rösch et al. (2004) [5] | 50 | 0 | No adverse events |




 PDF
PDF Citation
Citation Print
Print



 High risk
High risk XML Download
XML Download