INTRODUCTION
Table 1.
METHODS
RESULTS
Findings from Iwamuro et al. (2010) [22]
Table 2.
| Study | No. of patientsa) | EUS-BD technique | Technical success, EUS-BD (No., %) | Technical success, DuS (No., %) | Clinical success (oral intake, No., %) | Non-mortality-related rate of post-endoscopy adverse events (early or late) | Biliary stent and/or DuS dysfunction (No., %) | Subsequent need for surgery after stent dysfunction | Biliary stent type | Duodenal stent type |
|---|---|---|---|---|---|---|---|---|---|---|
| Iwamuro et al. (2010) [22] | 2 | EUS-CDS | 2/2 (100%) | 2/2 (100%) | 2/2 (100%) | Early: 1/2 (50%) | Biliary: 2/2 (100%)b) | 0% | PS (7 Fr 50 mm or 7 Fr 40 mm) | 8 cm × 20 mm SEMS (Niti-S; TaeWoong Medical, Seoul, Korea)c) |
| Late: 0/2 (0%) | ||||||||||
| Kawakubo et al. (2012) [23] | 2 | EUS-CDS | 2/2 (100%) | 2/2 (100%) | 2/2 (100%) | Early: 0/2 (0%) | Biliary: 0/2 (0%) | 0% | 7 Fr straight PS (Flexima; Boston Scientific, Marlborough, MA, USA) | Uncovered SEMS, WallFlex; covered SEMS (ComVi; TaeWoong Medical) |
| Late: 0/2 (0%) | ||||||||||
| Rebello et al. (2012) [24] & Artifon et al. (2013) [25] | 7 | EUS-CDS | 7/7 (100%) | 7/7 (100%) | 7/7 (100%) | Early: 0/7 (0%) | Biliary: 1/7 (14.3%) | 0% | Partially covered self-expandable metal stents (WallFlex) (8×60 mm; 10×60 mm; 10×80 mm) | SEMS (18×90 mm; 18×110 mm; 22×60 mm; 22×90 mm) |
| Late: 1/7 (14.3%) | ||||||||||
| Tonozuka et al. (2013) [26] | 4 | EUS-CDS; EUS-HGS | 4/4 (100%) | 4/4 (100%) | 4/4 (100%) | Early: 0/4 (0%) | Biliary: 0/2 (0%) | 0% | Not specified | 10 cm × 20 mm Niti-S; 6–9 cm × 22 mm WallFlexc) |
| Late: 2/4 (50%) | ||||||||||
| Sato et al. (2016) [2] | 17 | EUS-CDS; EUS-HGS | 17/17 (100%)c) | 17/17 (100%)d) | N/Ae) | Early: 5/43 (12%)f) | N/Ac) | 0% | EUS-CDS: | Uncovered Niti-S and WallFlex stents (6, 8 10, 12 cm × 20 or 22 mm) |
| Late: 3/43 (7%)f) | Fully covered stent (WallFlex) (4 and 6 cm × 10 mm | |||||||||
| EUS-HGS: | ||||||||||
| Fully covered stent (Niti-S) | ||||||||||
| Matsumoto et al. (2017) [12] | 19 | EUS-CDS; EUS-HGS | 19/19 (100%) | 19/19 (100%) | 19/19 (100%) | N/Ag) | DuS: 3/19 (15.8%); biliary stent by EUSBD: 8/19 (42.1%)h) | N/A | Covered 10 mm × 6 cm stents (WallFlex or Bonastent; Sewoon Medical, Seoul, Korea); PS (Flexima or Zimmon [Cook Medical, Winston-Salem, NC, USA]) | 20-mm covered SEMS (Niti-S); 20-mm and 22-mm uncovered SEMS (Niti-S or WallFlex) |
DuS, duodenal stent; EUS-BD, endoscopic ultrasound-guided biliary drainage; EUS-CDS, endoscopic ultrasound-guided choledochoduodenostomy; EUS-HGS, endoscopic ultrasound-guided hepaticogastrostomy; N/A, not available; PS, plastic stent; SEMS, self-expanding metal stent.
a) A total of 144 patients across 5 studies were analyzed; 44 patients were identified having underwent simultaneous EUS-BD and duodenal stenting for gastric outlet obstruction. Since our intent was to isolate only EUS-guided cases of double-stenting, data related to were interpreted and extracted from the associated articles.
d) Because no immediate complications were explicitly attributed to double-stenting in these cases, this was regarded as technical success. However, seven patients later required re-intervention; in re-intervention group, 6 of 7 cases underwent EUS-BD, and 5 of 7 cases were technically successful (71.4%). Re-intervention was not counted against immediate technical success; re-intervention was considered “stent dysfunction”. Because explicit correlation was not made for which of the re-interventions were attributed to the patients who underwent EUS-BD, a percentage could not be calculated.
f) Due to inability to exactly ascribe each individual case with respective outcomes, this limitation affected interpretation of the proportions. For instance, in the reporting of post-endoscopy adverse events from Sato et al., the proportions could only be derived from a cohort of 43 patients instead of 17 patients.[2]
Findings from Kawakubo et al. (2012) [23]
Findings from Rebello et al. (2012) [24] and Artifon et al. (2013) [25]
Findings from Tonozuka et al. (2013) [26]
Findings from Sato et al. (2016) [2]
Findings from Matsumoto et al. (2017) [12]
Double stenting
SUMMARY OF RESULTS FROM STUDIES
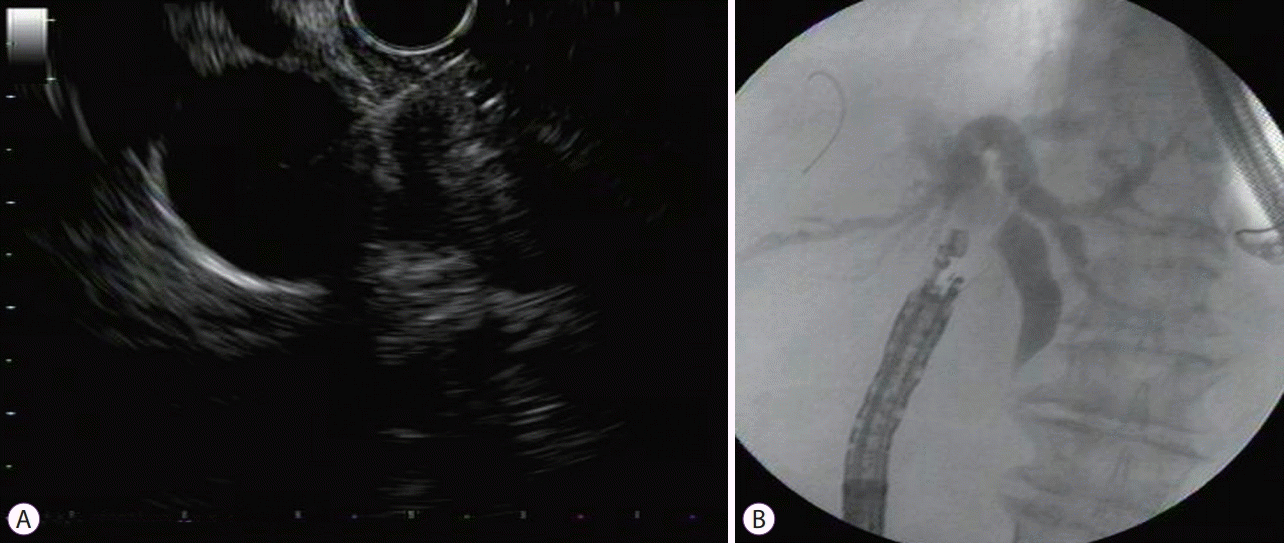
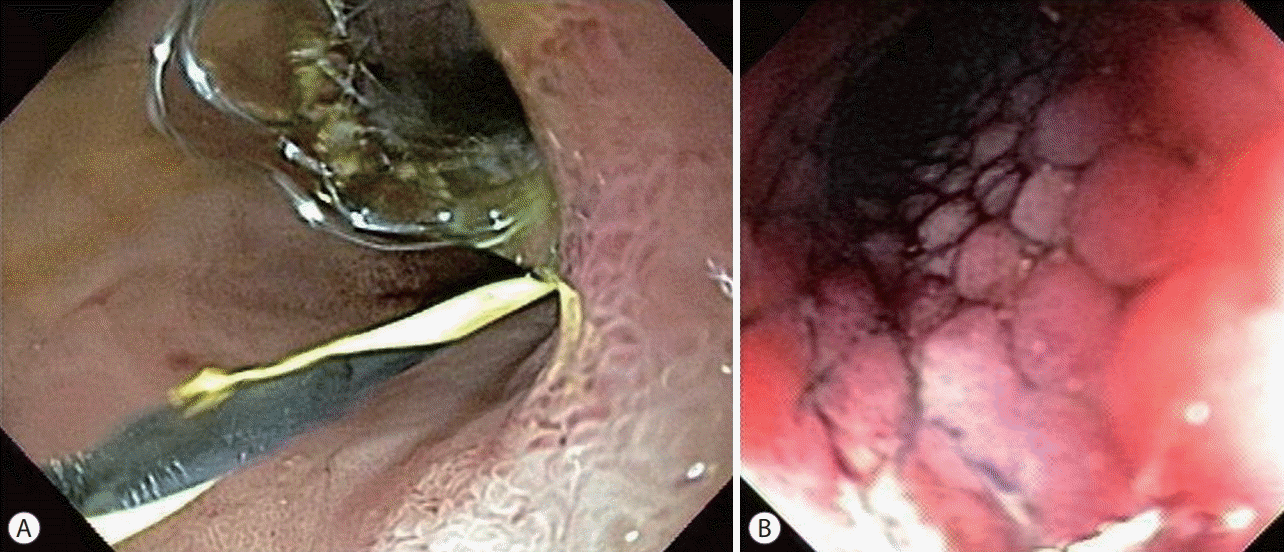
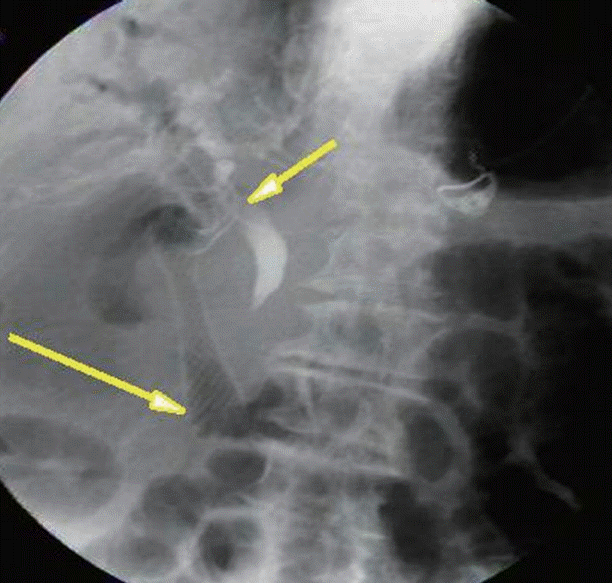
CASE IN POINT
DISCUSSION
CONCLUSIONS
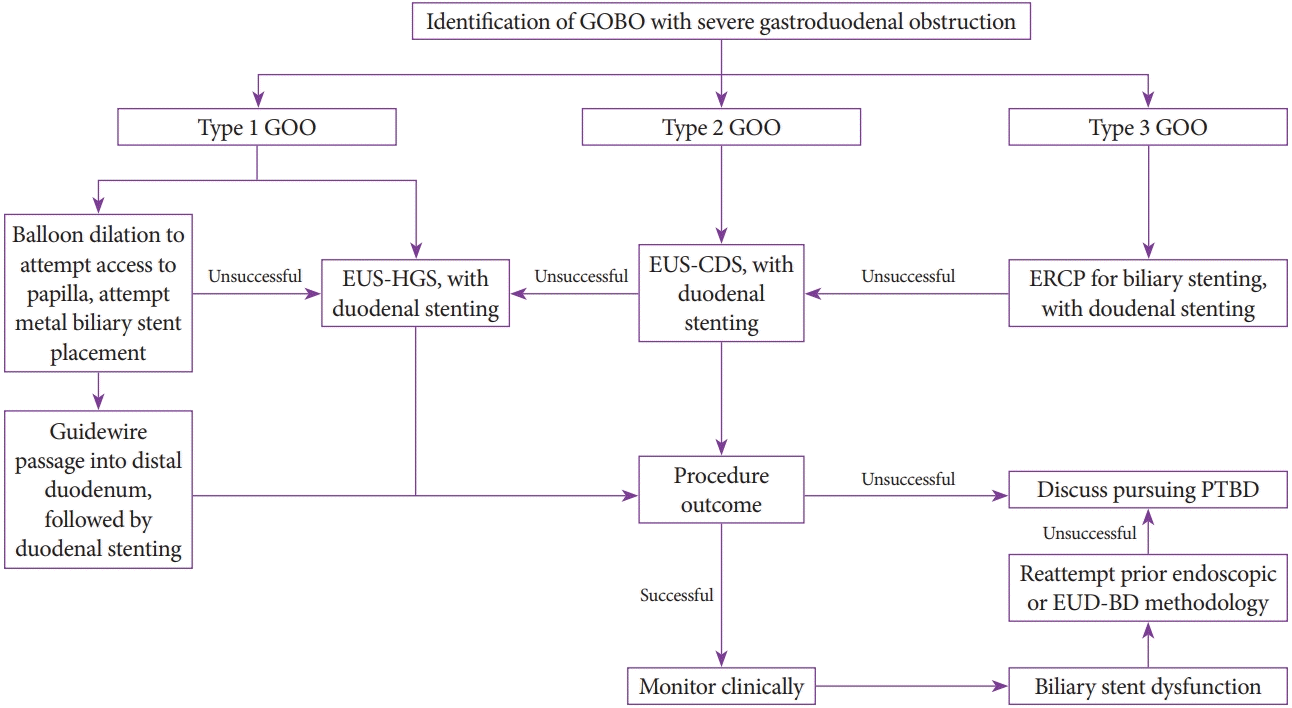 | Fig. 4.Proposed algorithm to determine the decision to pursue endoscopic ultrasound-guided biliary drainage (EUS-BD) and simultaneous duodenal stenting and management for initial biliary stent dysfunction. Accessibility to the papilla dictates the endoscopic methodology. After a successful endoscopy, patients should be monitored clinically, including for signs suggesting stent dysfunction. ERCP, endoscopic retrograde cholangiopancreatography; EUS-CDS, endoscopic ultrasound-guided choledochoduodenostomy; EUS-HGS, endoscopic ultrasound-guided hepaticogastrostomy; GOBO, gastric outlet and biliary obstruction; GOO, gastric outlet obstruction; PTBD, percutaneous transhepatic biliary drainage. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download