METHODS
Patients
We retrospectively reviewed the medical records of patients with histopathologically proven gastric GISTs or leiomyomas originating from the fourth layer who underwent EUS examinations at eight university hospitals (members of the Research Group for Endoscopic Ultrasound of the Korean Society of Gastrointestinal Endoscopy) between January 2005 and December 2015. For the immunohistochemical analysis, GISTs were defined as positive for c-KIT, while leiomyomas were defined as positive for desmin or smooth muscle actin.
We enrolled 361 patients (162 men and 199 women) with a mean age of 57 years (range, 26‒87 years), of whom 274 had GISTs and 87 had leiomyomas. The pathological findings were confirmed using tissues obtained surgically (306 patients [84.8%]), biopsy with endoscopic forceps (37 patients [10.2%]), EUS-guided fine-needle biopsy (EUS-FNB) (13 patients [3.6%]), or endoscopic resection (5 patients [1.4%]), including endoscopic mucosal resection or endoscopic submucosal dissection.
This study was performed in accordance with the ethical guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Konyang University Hospital (approval no. 2015-07-015).
Patient data
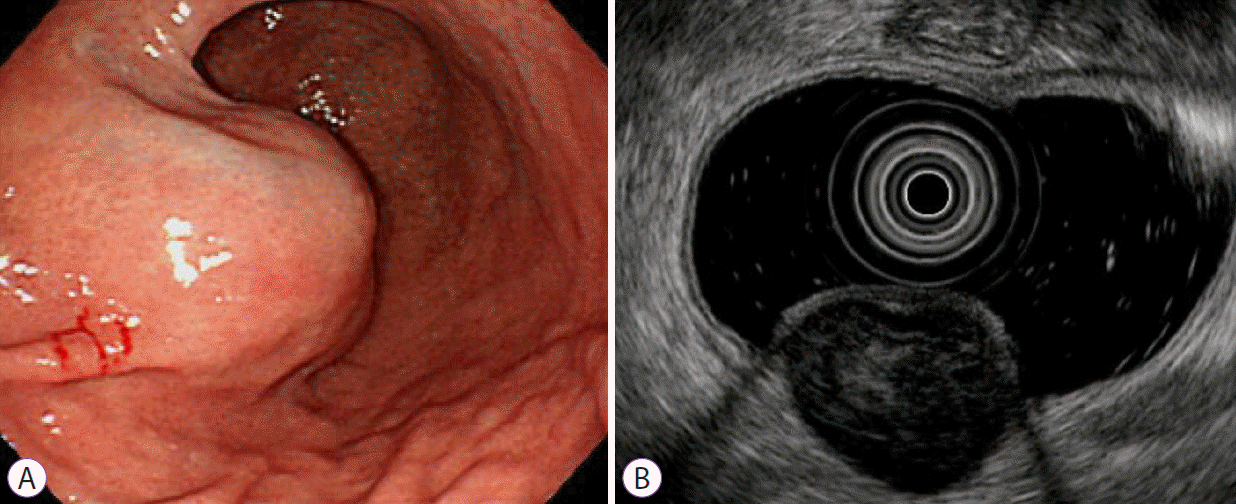
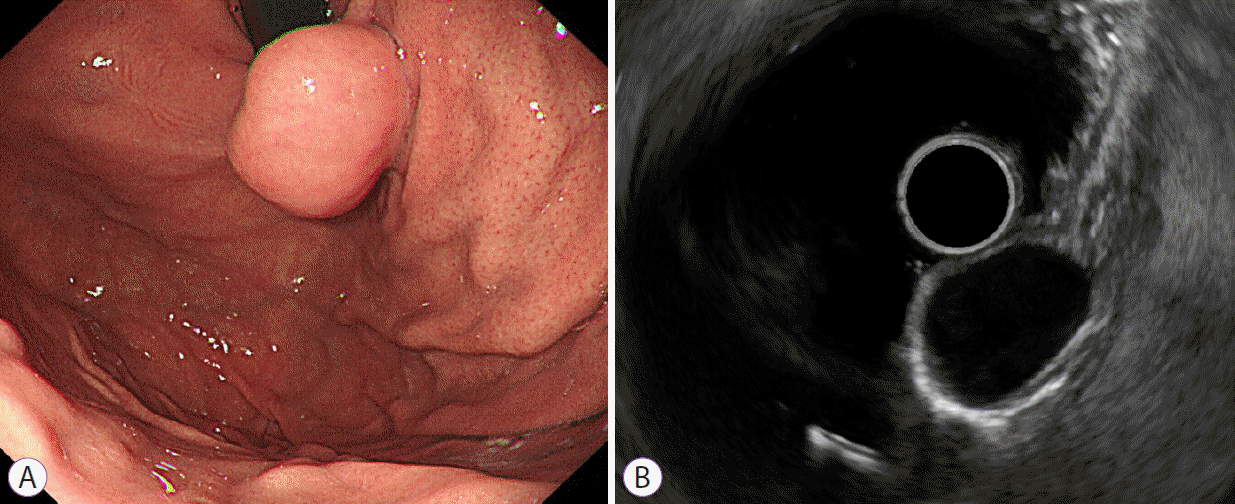
From the medical records including EGD and EUS images, we obtained data on patient demographics and tumor characteristics, including location (non-cardia, cardia), tumor shape (non-elongated, elongated), tumor growth pattern (endophytic, exophytic, mixed), and the presence of dimpling or ulcer, mucosal erythema, and lobulation. Data pertaining to maximal diameter, echogenicity (hyperechoic, isoechoic, hypoechoic), homogeneity (homogenous, heterogeneous), presence of anechoic spaces, presence of hyperechoic spots, and marginal regularity (regular, irregular) were recorded based on the EUS findings. Echogenicity was determined by comparison with the normal muscularis propria layer. The term “homogenous” was defined as more than 75% of the total area appearing homogenous.
The interpretation of EUS images involves many inter-observer differences. Therefore, we collected three typical cases from each hospital and selected 20 cases worthy of discussion. Two consensus meetings were held on the EUS findings in these cases. After consensus meetings, the cases from each hospital were interpreted independently by endosonographers according to the criteria determined at the meeting, and the results were collected.
Statistical analysis
In this study, we evaluated the inter-observer agreement of the five EUS findings using Fleiss’s kappa (ĸ) to compare their reliability. A ĸ value of 1.0 indicates perfect agreement. Values of ĸ>0.80 were considered “excellent,” 0.60‒0.79 were considered “good,” 0.40‒0.59 were considered “fair,” and <0.40 were considered “poor.”
Categorical variables were analyzed using the χ2 test or Fisher’s exact test. Continuous variables were analyzed using Student’s t-test or the Mann–Whitney U-test. Univariate binary logistic regression was used to assess the effectiveness of potential predictive factors in differentiating GISTs from leiomyomas in patients with SETs. A multivariate predictive model was then constructed using a backward stepwise variable elimination procedure including the predictive factors with values of p<0.1 in the univariate analysis.
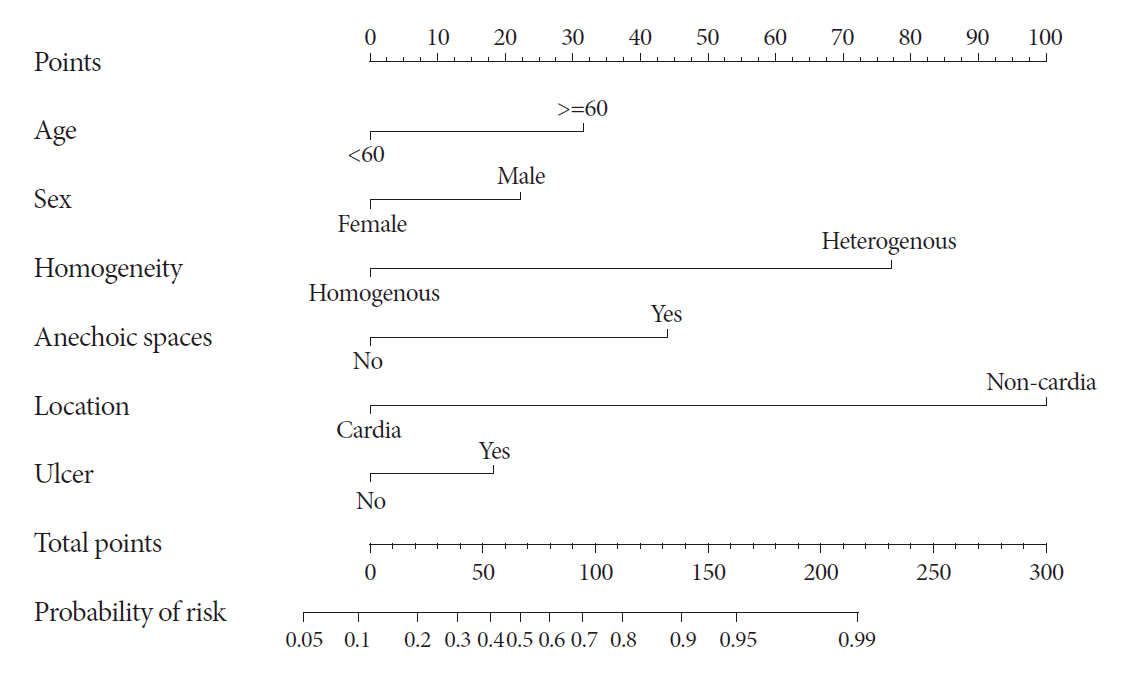
Receiver operating characteristic (ROC) curve analysis was performed to measure the discrimination performance in the development and internal validation sets. The reliability of the constructed model was internally validated using leave-one-out cross-validation. For practical application of the prediction model in the clinical field, a nomogram was constructed using the selected predictors.
Statistical significance was set at p<0.05. The statistical analyses were performed using R version 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria) and T&F program ver. 2.8 (YooJin BioSoft Co., Ltd, Goyang, Korea).
DISCUSSION
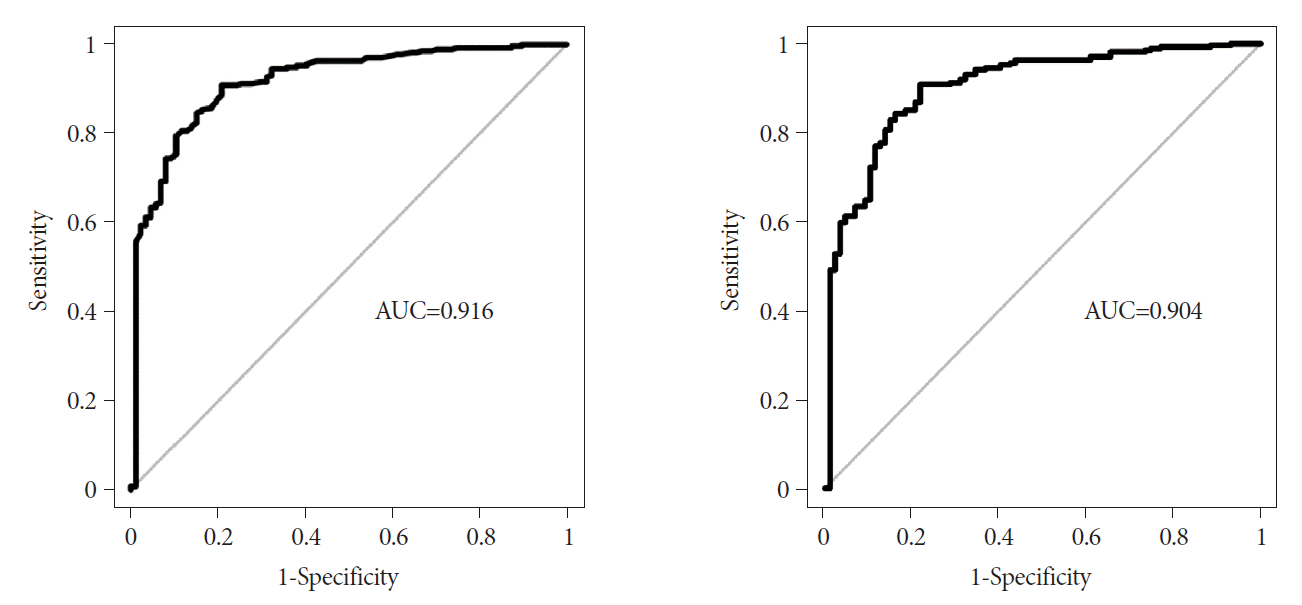
In this multicenter retrospective study, we aimed to investigate the predictive factors and develop a predictive risk score model and nomogram for differentiating gastric GISTs and leiomyomas based on EUS findings. The multivariate analysis identified heterogeneity, non-cardia, and older age as independent predictors of GISTs. The areas under the ROC curve of the predictive model using age, sex, and four EUS factors (heterogeneity, location, anechoic spaces, and dimpling or ulcer) were 0.916 (sensitivity, 0.908; specificity, 0.793) and 0.904 (sensitivity, 0.908; specificity, 0.782) in the development and internal validation sets, respectively.
EUS is the most valuable diagnostic tool for evaluating the layer of origin, size, internal echogenicity, margin, and echotexture of SETs [
10]. However, although several studies have aimed to diagnose SETs using EUS, the diagnostic accuracy was relatively low for the differential diagnosis of such tumors, particularly for those that originate from the muscularis propria layer [
8,
9,
11]. In addition, since the interpretation of EUS findings of gastric SETs has significant inter-observer variability, most studies of SETs have been single-center studies with small sample sizes [
12]. Thus, we here planned a large sample size. Additionally, two consensus meetings were held to overcome inter-observer variability in the analysis of the EUS images, which is considered the largest problem with this approach. The first interpretation was based on existing personal criteria. The ĸ values for inter-observer variability were very low (ĸ<0.40), and the agreement of the five EUS findings was poor. After the first meeting, the average ĸ value improved to 0.28, but the agreement of four EUS findings, except homogeneity, remained poor. Consequently, we conducted an additional consensus meeting. The average ĸ value of the third interpretation improved to 0.459 and the agreement was fair. The agreement for hyperechoic spots was good, whereas that for homogeneity and anechoic spaces was fair. Unfortunately, the agreement on the echogenicity and the marginal irregularity remained poor after the second meeting. To our knowledge, no report to date has evaluated the inter-observer agreement of EUS findings of gastric SETs. Despite these two meetings, the agreement improved only somewhat and not satisfactorily. Thus, we confirmed that the interpretation of EUS findings of gastric SETs has significant inter-observer variability.
In this study, GISTs were more common in the gastric antrum, body, and fundus. In contrast, leiomyomas were more common in the cardia. These results were similar to those reported in previous studies [
13,
14]. Most leiomyomas do not require surgery. In recent years, most SET operations have been performed using laparoscopic wedge resection. The cardia area is known to be a very difficult operation site because of the technical complexities of the procedure and complications such as gastroesophageal reflux or late stenosis [
14]. When considering surgery in SETs in the cardia, efforts for preoperative pathologic diagnosis, such as EUS-FNB, unroofing technique, and single-incision needle-knife biopsy, are needed to avoid unnecessary surgery.
In a previous study, homogeneity, echogenicity, the presence of hyperechogenic spots and anechoic spaces, older age, and presence of a marginal halo were reported EUS findings for differentiating GISTs from leiomyomas [
8,
15,
16]. In the present study, older age, tumor inhomogeneity, and non-cardia site were independent predictive factors for GISTs on multivariate analysis. In contrast, echogenicity, the presence of hyperechogenic spots and anechoic spaces, tumor shape, mucosal erythema, and marginal regularity were not helpful for predicting GISTs. In fact, among the important EUS findings, homogeneity was the only predictive factor.
The current study is the first to analyze the characteristics of tumor by size groups including tumors with 0.6–11.7 cm in size. In SETs smaller than 2 cm, age, sex, homogeneity, and tumor location were significant independent predictive factors for differentiating GISTs and leiomyomas. In tumors with 2−3.5 cm in size, age and homogeneity were significant, while in tumors larger than 3.5 cm, age, tumor shape, and hyperechoic spots were significant.
In the development set, the area under the ROC curve in gastric SETs smaller than 2.0 cm, 2.0–3.5 cm, and larger than 3.5 cm were 0.841, 0.812, and 0.968, respectively. We were mostly interested in gastric SETs with 2.0−3.5 cm in size, which decided whether to undergo surgery according to histopathology of SETs, but the discriminative ability of EUS was lowest for tumors of this size range.
Moreover, in the internal validation set, SETs smaller than 2.0 cm and with 2.0−3.5 cm in size showed areas under the curve similar to those of the development set. However, SETs larger than 3.5 cm had a lower area under the curve, which was most likely due to the small number of leiomyomas larger than 3.5 cm.
In the present study, we developed a predictive risk score model for differentiating gastric GISTs and leiomyomas that originate from the muscularis propria layer based on EUS findings. If the SETs were diagnosed as GISTs with a cutoff level of 0.708, the sensitivity, specificity, and accuracy of our model were 0.908, 0.793, and 0.881, respectively. This level of accuracy was noteworthy. Therefore, this model might be a complementary method to various other methods currently applied for discrimination of GISTs.
However, this study has some limitations. First, it was retrospective and used endosonographic images; therefore, there might have been selection bias. Second, several endoscopists independently interpreted the EUS findings of the gastric SETs. Although two consensus meetings were held to reduce inter-observer variability, agreement ended up just “fair”. Finally, this study included small SETs.
Despite these limitations, the present study is important because it involved a multicenter trial that used two consensus meetings to reduce inter-observer variability and included a relatively large number of patients with leiomyoma. In addition, no previous study performed an analysis by tumor size.
In conclusion, EUS features such as heterogeneity, non-cardia site, and older age were significant independent factors for differentiating gastric GISTs from leiomyomas. Additionally, the predictive model and nomogram developed in the present study, which uses age, sex, homogeneity, the presence of anechoic spaces, tumor location, and the presence of dimpling or ulcer, may be helpful for differentiating GISTs from leiomyomas.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download