1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.

2. Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015; 64:381–387.

3. Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012; 62:118–128.

4. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013; 381:400–412.

5. Arnold M, Laversanne M, Brown LM, Devesa SS, Bray F. Predicting the future burden of esophageal cancer by histological subtype: international trends in incidence up to 2030. Am J Gastroenterol. 2017; 112:1247–1255.

6. Wong A, Fitzgerald RC. Epidemiologic risk factors for Barrett’s esophagus and associated adenocarcinoma. Clin Gastroenterol Hepatol. 2005; 3:1–10.

7. Maitra I, Date RS, Martin FL. Towards screening Barrett’s oesophagus: current guidelines, imaging modalities and future developments. Clin J Gastroenterol. 2020; 13:635–649.

8. Gupta B, Kumar N. Worldwide incidence, mortality and time trends for cancer of the oesophagus. Eur J Cancer Prev. 2017; 26:107–118.

9. Nuytens F, Dabakuyo-Yonli TS, Meunier B, et al. Five-year survival outcomes of hybrid minimally invasive esophagectomy in esophageal cancer: results of the miro randomized clinical trial. JAMA Surg. 2021; 156:323–332.
10. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003; 349:2241–2252.

11. Huh CW, Jung DH, Kim JH, Ma DW, Youn YH, Park H. Clinical implication of endoscopic gross appearance in superficial esophageal squamous carcinoma: revisited. Surg Endosc. 2018; 32:367–375.

12. Higuchi K, Tanabe S, Koizumi W, et al. Expansion of the indications for endoscopic mucosal resection in patients with superficial esophageal carcinoma. Endoscopy. 2007; 39:36–40.

13. Choi JY, Park YS, Jung HY, et al. Feasibility of endoscopic resection in superficial esophageal squamous carcinoma. Gastrointest Endosc. 2011; 73:881–889, 889.e1- e2.

14. Nishizawa T, Suzuki H. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Cancers. 2020; 12:E2849.

15. Muto M, Minashi K, Yano T, et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010; 28:1566–1572.

16. Goda K, Dobashi A, Yoshimura N, et al. Narrow-band imaging magnifying endoscopy versus lugol chromoendoscopy with pink-color sign assessment in the diagnosis of superficial esophageal squamous neoplasms: a randomised noninferiority trial. Gastroenterol Res Pract. 2015; 2015:639462.

17. Hoffman A, Manner H, Rey JW, Kiesslich R. A guide to multimodal endoscopy imaging for gastrointestinal malignancy - an early indicator. Nat Rev Gastroenterol Hepatol. 2017; 14:421–434.

18. Mori M, Adachi Y, Matsushima T, Matsuda H, Kuwano H, Sugimachi K. Lugol staining pattern and histology of esophageal lesions. Am J Gastroenterol. 1993; 88:701–705.
19. Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: european society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy. 2015; 47:829–854.

20. Hashimoto CL, Iriya K, Baba ER, et al. Lugol’s dye spray chromoendoscopy establishes early diagnosis of esophageal cancer in patients with primary head and neck cancer. Am J Gastroenterol. 2005; 100:275–282.

21. Toriie S, Akasaka Y, Yamaguchi K, et al. New trial for endoscopical observation of esophagus by dye spraying method. G E N. 1976; 30:159–165.
22. Shiozaki H, Tahara H, Kobayashi K, et al. Endoscopic screening of early esophageal cancer with the Lugol dye method in patients with head and neck cancers. Cancer. 1990; 66:2068–2071.

23. Ina H, Shibuya H, Ohashi I, Kitagawa M. The frequency of a concomitant early esophageal cancer in male patients with oral and oropharyngeal cancer. Screening results using Lugol dye endoscopy. Cancer. 1994; 73:2038–2041.

24. Meyer V, Burtin P, Bour B, et al. Endoscopic detection of early esophageal cancer in a high-risk population: does Lugol staining improve videoendoscopy? Gastrointest Endosc. 1997; 45:480–484.

25. Shimizu Y, Omori T, Yokoyama A, et al. Endoscopic diagnosis of early squamous neoplasia of the esophagus with iodine staining: high-grade intra-epithelial neoplasia turns pink within a few minutes. J Gastroenterol Hepatol. 2008; 23:546–550.

26. Kondo H, Fukuda H, Ono H, et al. Sodium thiosulfate solution spray for relief of irritation caused by Lugol’s stain in chromoendoscopy. Gastrointest Endosc. 2001; 53:199–202.

27. Dubuc J, Legoux J-L, Winnock M, et al. Endoscopic screening for esophageal squamous-cell carcinoma in high-risk patients: a prospective study conducted in 62 French endoscopy centers. Endoscopy. 2006; 38:690–695.

28. Pech O. Lugol staining for early esophageal neoplasia: less is sometimes more. Gastrointest Endosc. 2020; 91:771–772.

29. Gotoda T, Kanzaki H, Okamoto Y, et al. Tolerability and efficacy of the concentration of iodine solution during esophageal chromoendoscopy: a double-blind randomized controlled trial. Gastrointest Endosc. 2020; 91:763–770.

30. Longcroft-Wheaton G, Brown J, Basford P, Cowlishaw D, Higgins B, Bhandari P. Duration of acetowhitening as a novel objective tool for diagnosing high risk neoplasia in Barrett’s esophagus: a prospective cohort trial. Endoscopy. 2013; 45:426–432.

31. Longcroft-Wheaton G, Duku M, Mead R, Poller D, Bhandari P. Acetic acid spray is an effective tool for the endoscopic detection of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2010; 8:843–847.
32. Pohl J, Pech O, May A, Manner H, Fissler-Eckhoff A, Ell C. Incidence of macroscopically occult neoplasias in Barrett’s esophagus: are random biopsies dispensable in the era of advanced endoscopic imaging? Am J Gastroenterol. 2010; 105:2350–2356.

33. Tholoor S, Bhattacharyya R, Tsagkournis O, Longcroft-Wheaton G, Bhandari P. Acetic acid chromoendoscopy in Barrett’s esophagus surveillance is superior to the standardized random biopsy protocol: results from a large cohort study (with video). Gastrointest Endosc. 2014; 80:417–424.

34. Coletta M, Sami SS, Nachiappan A, Fraquelli M, Casazza G, Ragunath K. Acetic acid chromoendoscopy for the diagnosis of early neoplasia and specialized intestinal metaplasia in Barrett’s esophagus: a meta-analysis. Gastrointest Endosc. 2016; 83:57–67.e1.

35. ASGE Technology Committee, Thosani N, Abu Dayyeh BK, et al. ASGE technology committee systematic review and meta-analysis assessing the ASGE preservation and incorporation of valuable endoscopic innovations thresholds for adopting real-time imaging-assisted endoscopic targeted biopsy during endoscopic surveillance of Barrett’s esophagus. Gastrointest Endosc. 2016; 83:684–698.e7.
36. Canto MI, Setrakian S, Willis J, et al. Methylene blue-directed biopsies improve detection of intestinal metaplasia and dysplasia in Barrett’s esophagus. Gastrointest Endosc. 2000; 51:560–568.

37. Wo JM, Ray MB, Mayfield-Stokes S, et al. Comparison of methylene blue-directed biopsies and conventional biopsies in the detection of intestinal metaplasia and dysplasia in Barrett’s esophagus: a preliminary study. Gastrointest Endosc. 2001; 54:294–301.

38. Ragunath K, Krasner N, Raman VS, Haqqani MT, Cheung WY. A randomized, prospective cross-over trial comparing methylene blue-directed biopsy and conventional random biopsy for detecting intestinal metaplasia and dysplasia in Barrett’s esophagus. Endoscopy. 2003; 35:998–1003.

39. Ngamruengphong S, Sharma VK, Das A. Diagnostic yield of methylene blue chromoendoscopy for detecting specialized intestinal metaplasia and dysplasia in Barrett’s esophagus: a meta-analysis. Gastrointest Endosc. 2009; 69:1021–1028.

40. Kara MA, Peters FP, Rosmolen WD, et al. High-resolution endoscopy plus chromoendoscopy or narrow-band imaging in Barrett’s esophagus: a prospective randomized crossover study. Endoscopy. 2005; 37:929–936.

41. Morita FHA, Bernardo WM, Ide E, et al. Narrow band imaging versus lugol chromoendoscopy to diagnose squamous cell carcinoma of the esophagus: a systematic review and meta-analysis. BMC Cancer. 2017; 17:54.

42. Nagami Y, Tominaga K, Machida H, et al. Usefulness of non-magnifying narrow-band imaging in screening of early esophageal squamous cell carcinoma: a prospective comparative study using propensity score matching. Am J Gastroenterol. 2014; 109:845–854.

43. Lee CT, Chang CY, Lee YC, et al. Narrow-band imaging with magnifying endoscopy for the screening of esophageal cancer in patients with primary head and neck cancers. Endoscopy. 2010; 42:613–619.

44. Ishihara R, Tanaka H, Iishi H, et al. Long-term outcome of esophageal mucosal squamous cell carcinoma without lymphovascular involvement after endoscopic resection. Cancer. 2008; 112:2166–2172.

45. Inoue H, Honda T, Nagai K, et al. Ultra-high magnification endoscopic observation of carcinoma in situ of the esophagus. Dig Endosc. 1997; 9:16–18.

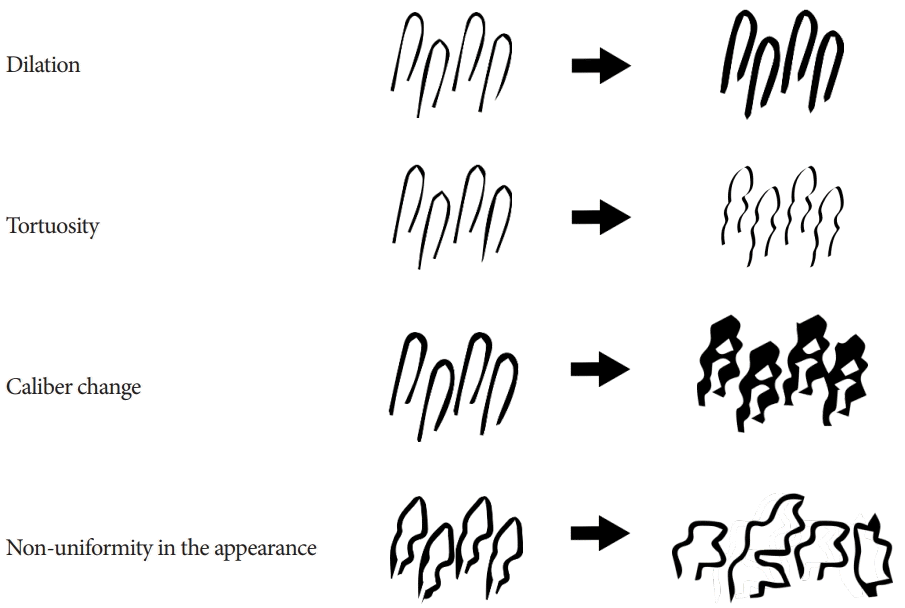
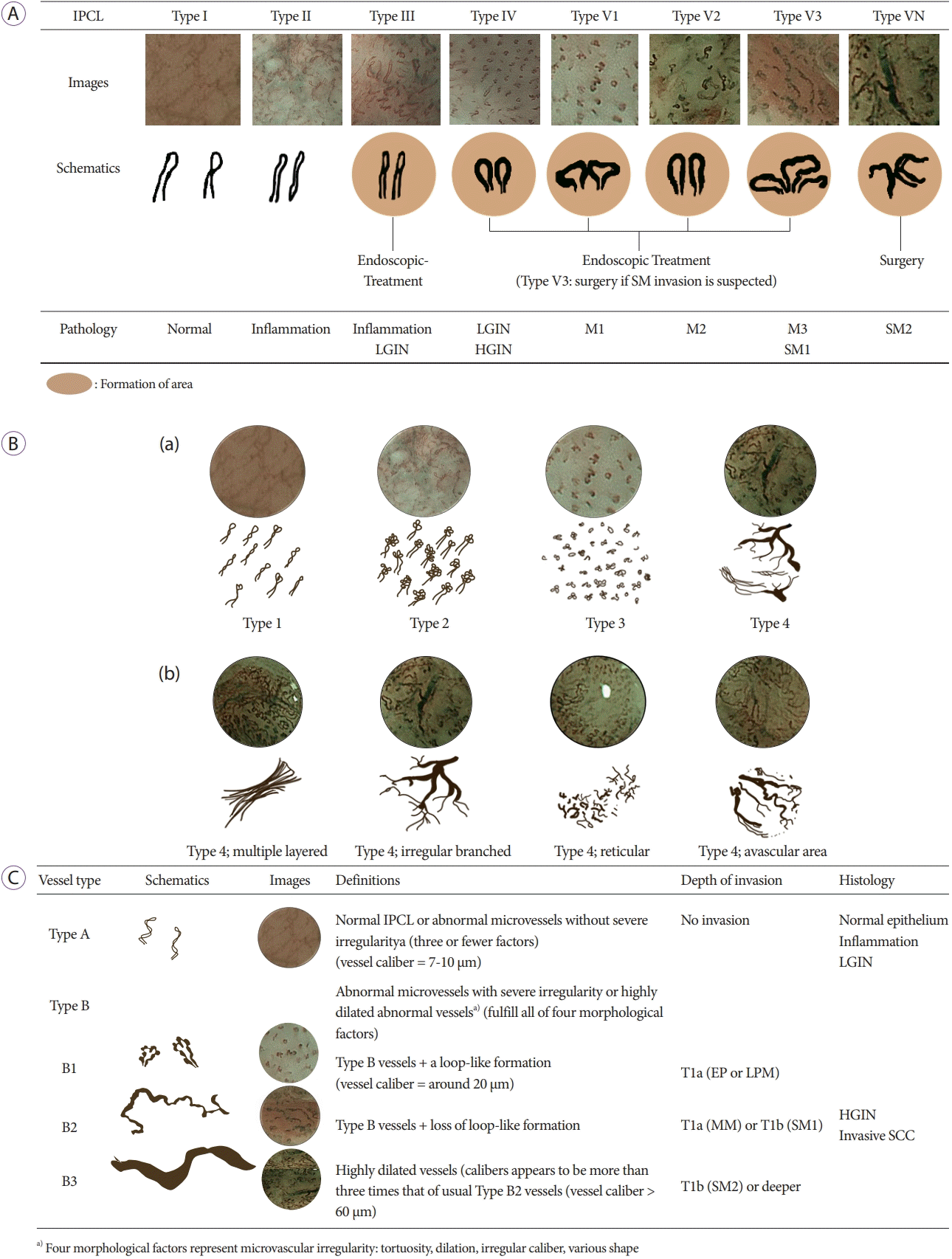
46. Inoue H, Kaga M, Ikeda H, et al. Magnification endoscopy in esophageal squamous cell carcinoma: a review of the intrapapillary capillary loop classification. Ann Gastroenterol. 2015; 28:41–48.
47. Inoue H, Honda T, Yoshida T, et al. Ultra-high magnification endoscopy of the normal esophageal mucosa. Dig Endosc. 1996; 8:134–138.

48. Arima M, Tada M, Arima H. Evaluation of microvascular patterns of superficial esophageal cancers by magnifying endoscopy. Esophagus. 2005; 2:191–197.

49. Goda K, Tajiri H, Ikegami M, et al. Magnifying endoscopy with narrow band imaging for predicting the invasion depth of superficial esophageal squamous cell carcinoma. Dis Esophagus. 2009; 22:453–460.

50. Katada C, Tanabe S, Wada T, et al. Retrospective assessment of the diagnostic accuracy of the depth of invasion by narrow band imaging magnifying endoscopy in patients with superficial esophageal squamous cell carcinoma. J Gastrointest Cancer. 2019; 50:292–297.

51. Oyama T, Inoue H, Arima M, et al. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: magnifying endoscopic classification of the Japan Esophageal Society. Esophagus. 2017; 14:105–112.

52. Kim SJ, Kim GH, Lee MW, et al. New magnifying endoscopic classification for superficial esophageal squamous cell carcinoma. World J Gastroenterol. 2017; 23:4416–4421.

53. Ishihara R, Takeuchi Y, Chatani R, et al. Prospective evaluation of narrow-band imaging endoscopy for screening of esophageal squamous mucosal high-grade neoplasia in experienced and less experienced endoscopists. Dis Esophagus. 2010; 23:480–486.
54. Gruner M, Denis A, Masliah C, et al. Narrow-band imaging versus Lugol chromoendoscopy for esophageal squamous cell cancer screening in normal endoscopic practice: randomized controlled trial. Endoscopy. 2021; 53:674–682.

55. Dobashi A, Goda K, Furuhashi H, et al. Diagnostic efficacy of dual-focus endoscopy with narrow-band imaging using simplified dyad criteria for superficial esophageal squamous cell carcinoma. J Gastroenterol. 2019; 54:501–510.

56. Goda K, Dobashi A, Yoshimura N, et al. Dual-focus versus conventional magnification endoscopy for the diagnosis of superficial squamous neoplasms in the pharynx and esophagus: a randomized trial. Endoscopy. 2016; 48:321–329.

57. Kastelein F, van Olphen SH, Steyerberg EW, Spaander MCW, Bruno MJ, ProBar-Study Group. Impact of surveillance for Barrett’s oesophagus on tumour stage and survival of patients with neoplastic progression. Gut. 2016; 65:548–554.

58. Sharma P, Bergman JJGHM, Goda K, et al. Development and validation of a classification system to identify high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus using narrow-band imaging. Gastroenterology. 2016; 150:591–598.

59. American Gastroenterological Association, Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American gastroenterological association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011; 140:1084–1091.

60. Sharma P, Hawes RH, Bansal A, et al. Standard endoscopy with random biopsies versus narrow band imaging targeted biopsies in Barrett’s oesophagus: a prospective, international, randomised controlled trial. Gut. 2013; 62:15–21.

61. Qumseya BJ, Wang H, Badie N, et al. Advanced imaging technologies increase detection of dysplasia and neoplasia in patients with Barrett’s esophagus: a meta-analysis and systematic review. Clin Gastroenterol Hepatol. 2013; 11:1562–1570.e1-e2.

62. Sharma P, Bansal A, Mathur S, et al. The utility of a novel narrow band imaging endoscopy system in patients with Barrett’s esophagus. Gastrointest Endosc. 2006; 64:167–175.

63. Kara MA, Ennahachi M, Fockens P, ten Kate FJW, Bergman JJGHM. Detection and classification of the mucosal and vascular patterns (mucosal morphology) in Barrett’s esophagus by using narrow band imaging. Gastrointest Endosc. 2006; 64:155–166.

64. Singh R, Anagnostopoulos GK, Yao K, et al. Narrow-band imaging with magnification in Barrett’s esophagus: validation of a simplified grading system of mucosal morphology patterns against histology. Endoscopy. 2008; 40:457–463.

65. Silva FB, Dinis-Ribeiro M, Vieth M, et al. Endoscopic assessment and grading of Barrett’s esophagus using magnification endoscopy and narrow-band imaging: accuracy and interobserver agreement of different classification systems (with videos). Gastrointest Endosc. 2011; 73:7–14.

66. Lipman G, Bisschops R, Sehgal V, et al. Systematic assessment with I-SCAN magnification endoscopy and acetic acid improves dysplasia detection in patients with Barrett’s esophagus. Endoscopy. 2017; 49:1219–1228.

67. Pohl J, May A, Rabenstein T, Pech O, Ell C. Computed virtual chromoendoscopy: a new tool for enhancing tissue surface structures. Endoscopy. 2007; 39:80–83.

68. Osawa H, Yamamoto H, Yamada N, et al. Diagnosis of endoscopic Barrett’s esophagus by transnasal flexible spectral imaging color enhancement. J Gastroenterol. 2009; 44:1125–1132.

69. Pohl J, May A, Rabenstein T, et al. Comparison of computed virtual chromoendoscopy and conventional chromoendoscopy with acetic acid for detection of neoplasia in Barrett’s esophagus. Endoscopy. 2007; 39:594–598.

70. Osawa H, Yamamoto H. Present and future status of flexible spectral imaging color enhancement and blue laser imaging technology. Dig Endosc. 2014; 26 Suppl 1:105–115.

71. Li YX, Shen L, Yu HG, Luo HS, Yu JP. Fujinon intelligent color enhancement for the diagnosis of early esophageal squamous cell carcinoma and precancerous lesion. Turk J Gastroenterol. 2014; 25:365–369.

72. Codipilly DC, Qin Y, Dawsey SM, et al. Screening for esophageal squamous cell carcinoma: recent advances. Gastrointest Endosc. 2018; 88:413–426.

73. Sharma P, Savides TJ, Canto MI, et al. The American Society for Gastrointestinal Endoscopy PIVI (preservation and incorporation of valuable endoscopic innovations) on imaging in Barrett’s esophagus. Gastrointest Endosc. 2012; 76:252–254.

74. Hoffman A, Korczynski O, Tresch A, et al. Acetic acid compared with i-scan imaging for detecting Barrett’s esophagus: a randomized, comparative trial. Gastrointest Endosc. 2014; 79:46–54.

75. Diao W, Huang X, Shen L, Zeng Z. Diagnostic ability of blue laser imaging combined with magnifying endoscopy for early esophageal cancer. Dig Liver Dis. 2018; 50:1035–1040.

76. Tomie A, Dohi O, Yagi N, et al. Blue laser imaging-bright improves endoscopic recognition of superficial esophageal squamous cell carcinoma. Gastroenterol Res Pract. 2016; 2016:6140854.

77. Kobayashi K, Miyahara R, Funasaka K, et al. Color information from linked color imaging is associated with invasion depth and vascular diameter in superficial esophageal squamous cell carcinoma. Dig Endosc. 2020; 32:65–73.

78. Nakamura K, Urabe Y, Oka S, et al. Usefulness of linked color imaging in the early detection of superficial esophageal squamous cell carcinomas. Esophagus. 2021; 18:118–124.

79. de Groof AJ, Swager AF, Pouw RE, et al. Blue-light imaging has an additional value to white-light endoscopy in visualization of early Barrett’s neoplasia: an international multicenter cohort study. Gastrointest Endosc. 2019; 89:749–758.

80. Tokunaga M, Matsumura T, Ishikawa K, et al. The efficacy of linked color imaging in the endoscopic diagnosis of barrett’s esophagus and esophageal adenocarcinoma. Gastroenterol Res Pract. 2020; 2020:9604345.

81. Takeda T, Nagahara A, Ishizuka K, et al. Improved visibility of Barrett’s esophagus with linked color imaging: inter- and intra-rater reliability and quantitative analysis. Digestion. 2018; 97:183–194.

82. de Groof AJ, Fockens KN, Struyvenberg MR, et al. Blue-light imaging and linked-color imaging improve visualization of Barrett’s neoplasia by nonexpert endoscopists. Gastrointest Endosc. 2020; 91:1050–1057.

83. Subramaniam S, Kandiah K, Schoon E, et al. Development and validation of the international blue light imaging for Barrett’s neoplasia classification. Gastrointest Endosc. 2020; 91:310–320.

84. Ohmori M, Ishihara R, Aoyama K, et al. Endoscopic detection and differentiation of esophageal lesions using a deep neural network. Gastrointest Endosc. 2020; 91:301–309.e1.

85. Fukuda H, Ishihara R, Kato Y, et al. Comparison of performances of artificial intelligence versus expert endoscopists for real-time assisted diagnosis of esophageal squamous cell carcinoma (with video). Gastrointest Endosc. 2020; 92:848–855.

86. Guo L, Xiao X, Wu C, et al. Real-time automated diagnosis of precancerous lesions and early esophageal squamous cell carcinoma using a deep learning model (with videos). Gastrointest Endosc. 2020; 91:41–51.

87. Everson M, Herrera L, Li W, et al. Artificial intelligence for the real-time classification of intrapapillary capillary loop patterns in the endoscopic diagnosis of early oesophageal squamous cell carcinoma: a proof-of-concept study. United European Gastroenterol J. 2019; 7:297–306.

88. Shimamoto Y, Ishihara R, Kato Y, et al. Real-time assessment of video images for esophageal squamous cell carcinoma invasion depth using artificial intelligence. J Gastroenterol. 2020; 55:1037–1045.

89. van der Sommen F, Zinger S, Curvers WL, et al. Computer-aided detection of early neoplastic lesions in Barrett’s esophagus. Endoscopy. 2016; 48:617–624.

90. Ebigbo A, Mendel R, Probst A, et al. Computer-aided diagnosis using deep learning in the evaluation of early oesophageal adenocarcinoma. Gut. 2019; 68:1143–1145.

91. Hashimoto R, Requa J, Dao T, et al. Artificial intelligence using convolutional neural networks for real-time detection of early esophageal neoplasia in Barrett’s esophagus (with video). Gastrointest Endosc. 2020; 91:1264–1271.e1.

92. Struyvenberg MR, de Groof AJ, van der Putten J, et al. A computer-assisted algorithm for narrow-band imaging-based tissue characterization in Barrett’s esophagus. Gastrointest Endosc. 2021; 93:89–98.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download