Abstract
Considering its contribution to reducing colorectal cancer morbidity and mortality, the most important task of colonoscopy is to find all existing polyps. Moreover, the accurate detection of existing polyps determines the risk of colorectal cancer morbidity and is an important factor in deciding the appropriate surveillance program for patients. Image-enhanced endoscopy is an easy-to-use modality with improved lesion detection. Linked color imaging (LCI) and blue laser/light imaging (BLI) are useful modalities for improving colonoscopy quality. Each mode has unique optical features; therefore, their intended use differs. LCI contributes to improved polyp detection due to its brightness and high color contrast between the lesion and normal mucosa, while BLI contributes to the characterization of detected polyps by evaluating the vessel and surface patterns of detected lesions. The proper use of these observation modes allows for more efficient endoscopic diagnosis. Moreover, recent developments in artificial intelligence will soon change the clinical practice of colonoscopy and this system will provide an efficient education modality for novice endoscopists.
Colorectal cancer (CRC) screening is a crucial for preventing CRC. Colonoscopy is a beneficial screening test because it can reduce both mortality and the incidence of CRC [1]. However, deficiencies such as missed lesions are unavoidable. The factors associated with missed lesions include the colonoscopy preparation, anatomical features of the colon, endoscopist experience, and clinicopathological features of the polyps. In terms of the clinicopathological features of polyps, flat-type lesions and sessile serrated lesions are generally considered more difficult to detect than protruding polyps.
Third-eye colonoscopy, wide-angle colonoscopy, and transparent hood have improved polyp detection to overcome these issues [2]. These modalities allow the visualization of polyps located on behind-folds and decrease adenomas miss rates. However, preparing independent tools makes the application of these advances less practical for use in daily clinical practice.
Image-enhanced endoscopy (IEE), such as linked color imaging (LCI) and blue laser/light imaging (BLI), is also reportedly useful in improving polyp detection. The benefits of IEE in clinical practice include convenience, selectivity according to the endoscopist’s preference, and lack of interference with maneuverability.
This review outlines the basic knowledge on LCI and BLI and the efficacy of these modalities for polyp detection in daily colonoscopy.
Almost a decade has passed since a new endoscopic system (LASEREO; Fujifilm Co., Tokyo, Japan) using light amplification by stimulated emission of radiation (laser) became available in daily practice. This system has a unique illumination feature that uses two lasers of different wavelengths (Fig. 1) [3,4]. In addition, another novel endoscopic system (ELUXEO; Fujifilm Co., Tokyo, Japan) using a light-emitting diode (LED) is also now available. This system has four independently controlled LED colors (Fig. 1).
The most prominent feature of colonoscopy using LCI is the brighter field of view compared to white light imaging (WLI) or BLI (Fig. 2). The inside of the colonic lumen is dark, as light does not reach this area; thus brighter conditions improve the identification of colonic polyps. In addition, color contrast between the lesion and normal mucosa is also critical for improving the detection of colonic polyps. Most polyps are shown in red, while sessile serrated lesions are white; LCI makes the red regions redder and white regions whiter. This feature makes it easier to identify polyps by emphasizing these characteristics compared to WLI observations.
In their prospective randomized study, Min et al. reported a higher polyp detection (91% vs. 73%, respectively, p<0.001) and per-patient adenoma detection (37% vs. 28%, respectively; 95% confidence interval, 2.41%-19.41%) rates for LCI compared to WLI [5]. Paggi et al. reported a reduction in the miss rate of neoplastic lesions in the right-side colon due to partial tandem colonoscopy in a randomized controlled trial (RCT). Double inspection of the LCI-WLI and WLI-LCI groups and right colon inspection performed using LCI or WLI for the first pass and the opposite mode for the second pass resulted in adenoma miss rates of 11.8% and 30.6%, respectively (p<0.001) [6]. Kudo et al. reported a two-fold higher detection of flat-type polyps (generally considered more difficult to detect than polypoid-type polyps) per patient for LCI compared to WLI (2.9±3.0 vs. 1.2±1.6, p=0.045) [7].
In addition to these clinical studies, Shinozaki et al. performed a meta-analysis on the detectability of colonic polyps using LCI and WLI and reported superior polyp and adenoma detection rates, as well as the detection of previously missed polyps, for LCI [8].
In general, endoscopists observe the colonic lumen to detect polyps during the withdrawal phase after cecal intubation. However, the shape of the sigmoid colon often changes between the insertion and withdrawal phases, which may result in different blind areas. Therefore, we examined the significance of polyp detection during the insertion phase using LCI [9]. In this study, the clinical outcomes were compared between patients who alternatively underwent colonoscopy with either LCI or WLI for insertion. The results showed that 16% of lesions located in the sigmoid colon were detected only during the insertion phase in the LCI group, whereas no such lesions were detected during the insertion phase in the WLI group. Hence, LCI may provide additional value by improving polyp detection, especially for sigmoid colon polyps, during the insertion phase.
Although the BLI mode, which has two types of lasers with wavelengths of 410 nm and 450 nm, is useful for diagnosing colorectal polyps, it is generally considered disadvantageous for polyp detection due to its darkness. The BLI-bright mode, which uses a higher-power 450 nm wavelength compared to the BLI mode, was developed to overcome this disadvantage. The BLI-bright mode maintained adequate brightness and contrast even at longer distances compared to the BLI-mode [10]; therefore, it might be helpful in polyp detection. Accordingly, most subsequent studies on polyp detection have been conducted using the BLI-bright mode.
Three randomized controlled trials (RCT) evaluated the ability of BLI to detect colorectal polyps. Ikematsu et al. used a xenon light source and reported a significantly higher mean number of adenomas per patient by BLI compared to WLI (1.27±1.73 vs. 1.01±1.36, p=0.008) [11]. Another RCT reported a lower adenoma miss rate for the BLI-bright mode with a laser light source compared to that of WLI [12]. The study randomized patients into tandem colonoscopy with BLI followed by WLI (BLI-WLI group) or WLI followed by WLI (WLI-WLI group) groups. The adenoma miss rate was 1.6% in the BLI-WLI group and 10.0% in the WLI-WLI group (p=0.001). A recent RCT comparing polyp detectability between BLI-bright and WLI with a laser light source in Singapore [13] randomized the patients to either the BLI-bright or WLI groups on withdrawal. The polyp detection rates for BLI and WLI were 59.8% and 40.0% (p=0.008) and the adenoma detection rates were 46.2% and 27.8% (p=0.010), respectively. Although the parameters and limitations are diverse in the above studies, their findings suggest the superiority of the BLI-bright mode for polyp detection.
Regarding the differential diagnosis for histology, a European group has established the BLI Adenoma Serrated International Classification (BASIC) system for the diagnosis of colorectal polyps using BLI with a xenon light source with and without optical magnification [14]. In 2019, Subramarian et al. validated the diagnostic performance of the BASIC classification before and after training using 45 small polyp images [15]. The overall diagnostic accuracy with BLI improved from 87% to 94% with adequate training (p<0.001). Later, a multicenter prospective study conducted in Italy evaluated the validity of the BASIC classification for diminutive colorectal polyps [16]. They studied 748 colorectal polyps in 333 participants and reported an overall diagnostic accuracy of 88.5% in those evaluated with high confidence. They also compared the BLI-directed and histology-directed surveillance intervals, reporting that BLI-directed surveillance allowed appropriate surveillance for 90% of patients according to the United States Multi-Society Task Force criteria [17] and 96% of patients according to the European Society of Gastrointestinal Endoscopy criteria [18]. The usefulness of the BASIC criteria was also validated in the United States [19].
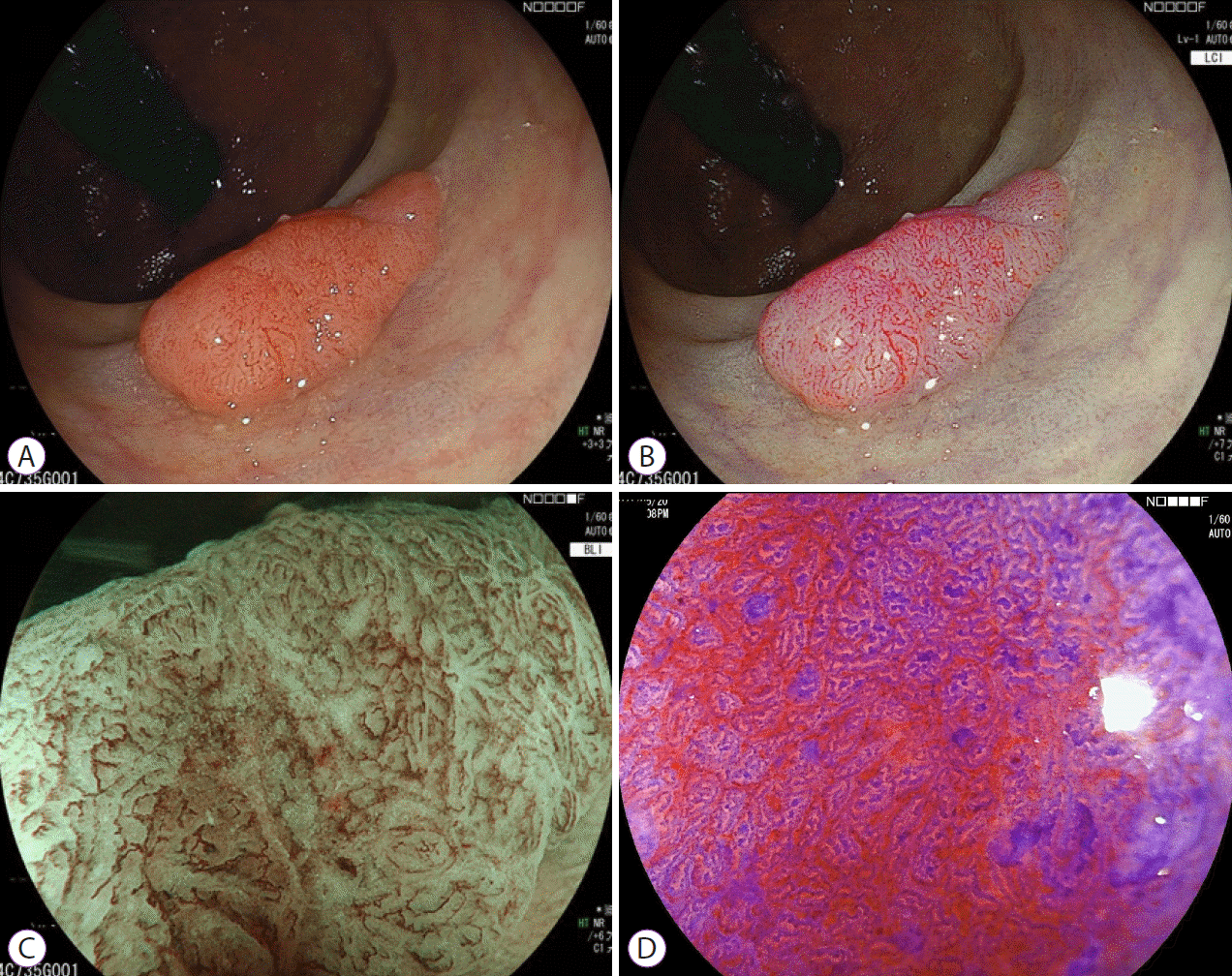
Depth diagnosis is crucial in deciding the treatment plan for superficial colorectal tumors. Magnifying chromoendoscopy with crystal violet staining is considered the gold standard for depth diagnosis of superficial colorectal tumors that cannot be determined by magnifying observation of vessels or surface patterns using narrow-band imaging (NBI) [20]. The diagnostic accuracy of pit pattern analysis was significantly higher than that with image-enhanced endoscopy [21]. A retrospective study from Japan assessed BLI with a laser light source for the diagnosis of the invasion depth of colorectal neoplasms with NBI classification (Hiroshima classification) [22]. They showed that the diagnostic accuracy of BLI magnification (74.0%), was comparable to that of NBI magnification (77.8%). The same group has also reported that BLI without magnification improved the differentiation between neoplastic and non-neoplastic polyps for small lesions (<10 mm) compared to WLI (accuracy rate: 95.2% vs. 83.2%, p=0.004) [23]. Nakano et al. investigated the diagnostic accuracy of BLI magnification with a laser light source versus pit pattern [24]. The accuracies of differentiation between neoplastic and non-neoplastic lesions were 98.4% and 98.7% for BLI magnification and pit pattern, respectively. However, the accuracy of BLI magnification for mucosal to shallow submucosal borderline invasive lesions was unfavorable. Only half of the lesions diagnosed as type C2 (Hiroshima classification; indicating mucosal to shallow submucosal invasion) by BLI magnification were consistent with the pathological diagnosis, whereas the pit pattern generated an accurate diagnosis in 72.2% of cases. Therefore, the authors concluded that most polyps could be diagnosed properly by BLI magnification but suggested adding pit pattern assessment to evaluate borderline lesions (Fig. 3).
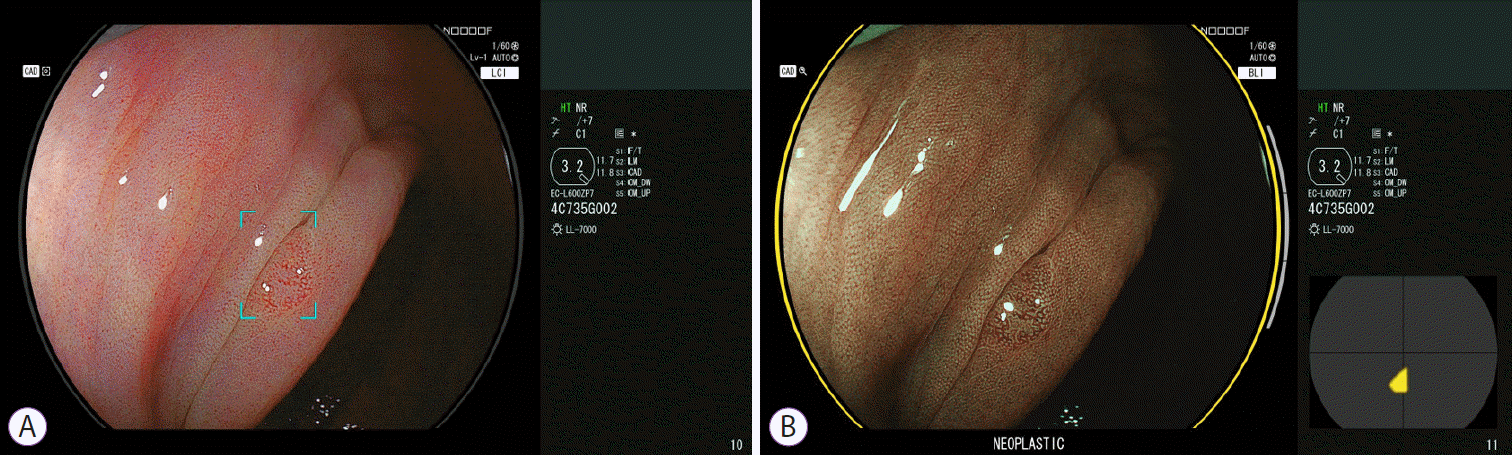
The recent development of artificial intelligence in colonoscopy has been remarkable. Currently, computer-aided diagnosis (CAD) is available not only for polyp detection but also for differentiating neoplasms from non-neoplasms. In terms of the assessments of the performance of the CAD systems, the experimental phase is complete and clinical trials have progressed rapidly. The CAD-EYE system from FUJIFILM was recently launched commercially and has been approved in Europe [25] and Japan. This system was developed to detect colorectal polyps (CADe system) and characterize the detected polyps (CADx system) (Fig. 4). Both the CADe and CADx systems provide WLI and BLI modes. The CADe system can accurately detect colon polyps with a sensitivity exceeding 90%, while the CADx system can predict the differential diagnosis with an overall accuracy of 90% (unpublished data). Moreover, the CADx system can operate without magnifying the observations. Hence, it saves the time required to perform magnifying observations in colonoscopy screening and helps novice endoscopists to learn magnifying observations. While not yet perfect, the CAD system will be a modality that changes the clinical practice of colonoscopy screening.
Based on the optical characteristics of LCI and BLI, because of its better visibility, LCI should be used during the insertion and withdrawal phases of colonoscopy, while BLI should be used to evaluate the characteristics of detected polyps and differentiate between neoplastic and non-neoplastic polyps. We believe that this process is the most efficient method for general colonoscopy according to existing evidence from clinical research.
REFERENCES
1. Pan J, Xin L, Ma YF, Hu LH, Li ZS. Colonoscopy reduces colorectal cancer incidence and mortality in patients with non-malignant findings: a meta-analysis. Am J Gastroenterol. 2016; 111:355–365.

2. Brand EC, Dik VK, van Oijen MGH, Siersema PD. Missed adenomas with behind-folds visualizing colonoscopy technologies compared with standard colonoscopy: a pooled analysis of 3 randomized back-to-back tandem colonoscopy studies. Gastrointest Endosc. 2017; 86:376–385.e2.

3. Osawa H, Miura Y, Takezawa T, et al. Linked color imaging and blue laser imaging for upper gastrointestinal screening. Clin Endosc. 2018; 51:513–526.

4. Yoshida N, Dohi O, Inoue K, et al. Blue laser imaging, blue light imaging, and linked color imaging for the detection and characterization of colorectal tumors. Gut Liver. 2019; 13:140–148.

5. Min M, Deng P, Zhang W, Sun X, Liu Y, Nong B. Comparison of linked color imaging and white-light colonoscopy for detection of colorectal polyps: a multicenter, randomized, crossover trial. Gastrointest Endosc. 2017; 86:724–730.

6. Paggi S, Mogavero G, Amato A, et al. Linked color imaging reduces the miss rate of neoplastic lesions in the right colon: a randomized tandem colonoscopy study. Endoscopy. 2018; 50:396–402.

7. Kudo T, Horiuchi A, Kyodo R, et al. Linked colour imaging versus white-light colonoscopy for the detection of flat colorectal lesions: a randomized controlled trial. Colorectal Dis. 2021; 23:1414–1420.

8. Shinozaki S, Kobayashi Y, Hayashi Y, et al. Colon polyp detection using linked color imaging compared to white light imaging: systematic review and meta-analysis. Dig Endosc. 2020; 32:874–881.

9. Sakamoto T, Tomizawa Y, Cho H, et al. Additional value of linked color imaging in colonoscopy: a retrospective study. Endosc Int Open. 2019; 7:E1448–E1454.

10. Kaneko K, Oono Y, Yano T, et al. Effect of novel bright image enhanced endoscopy using blue laser imaging (BLI). Endosc Int Open. 2014; 2:E212–E219.

11. Ikematsu H, Sakamoto T, Togashi K, et al. Detectability of colorectal neoplastic lesions using a novel endoscopic system with blue laser imaging: a multicenter randomized controlled trial. Gastrointest Endosc. 2017; 86:386–394.

12. Shimoda R, Sakata Y, Fujise T, et al. The adenoma miss rate of blue-laser imaging vs. white-light imaging during colonoscopy: a randomized tandem trial. Endoscopy. 2017; 49:186–190.

13. Ang TL, Li JW, Wong YJ, et al. A prospective randomized study of colonoscopy using blue laser imaging and white light imaging in detection and differentiation of colonic polyps. Endosc Int Open. 2019; 7:E1207–E1213.

14. Bisschops R, Hassan C, Bhandari P, et al. BASIC (BLI Adenoma Serrated International Classification) classification for colorectal polyp characterization with blue light imaging. Endoscopy. 2018; 50:211–220.

15. Subramaniam S, Hayee B, Aepli P, et al. Optical diagnosis of colorectal polyps with blue light imaging using a new international classification. United European Gastroenterol J. 2019; 7:316–325.

16. Rondonotti E, Hassan C, Andrealli A, et al. Clinical validation of BASIC classification for the resect and discard strategy for diminutive colorectal polyps. Clin Gastroenterol Hepatol. 2020; 18:2357–2365.e4.

17. Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012; 143:844–857.

18. Hassan C, Quintero E, Dumonceau JM, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2013; 45:842–851.

19. Desai M, Kennedy K, Aihara H, et al. External validation of blue light imaging (BLI) criteria for the optical characterization of colorectal polyps by endoscopy experts. J Gastroenterol Hepatol. 2021; Apr. 30. [Epub]. https://doi.org/10.1111/jgh.15529.

20. Sakamoto T, Nakajima T, Matsuda T, et al. Comparison of the diagnostic performance between magnifying chromoendoscopy and magnifying narrow-band imaging for superficial colorectal neoplasms: an online survey. Gastrointest Endosc. 2018; 87:1318–1323.

21. Sakamoto T, Inoki K, Takamaru H, et al. Efficacy of linked colour imaging in magnifying chromoendoscopy with crystal violet staining: a pilot study. Int J Colorectal Dis. 2019; 34:1341–1344.

22. Yoshida N, Hisabe T, Inada Y, et al. The ability of a novel blue laser imaging system for the diagnosis of invasion depth of colorectal neoplasms. J Gastroenterol. 2014; 49:73–80.

23. Yoshida N, Yagi N, Inada Y, et al. Ability of a novel blue laser imaging system for the diagnosis of colorectal polyps. Dig Endosc. 2014; 26:250–258.

24. Nakano A, Hirooka Y, Yamamura T, et al. Comparison of the diagnostic ability of blue laser imaging magnification versus pit pattern analysis for colorectal polyps. Endosc Int Open. 2017; 5:E224–E231.

25. Weigt J, Repici A, Antonelli G, et al. Performance of a new integrated computer-assisted system (CADe/CADx) for detection and characterization of colorectal neoplasia. Endoscopy. 2021; 1. 25. [Epub]. https://doi.org/10.1055/a-1372-0419.

Fig. 1.
Wavelength differences among the three observation modes. (A) White light imaging (WLI). (B) Blue laser/light imaging (BLI). (C) Linked color imaging (LCI).
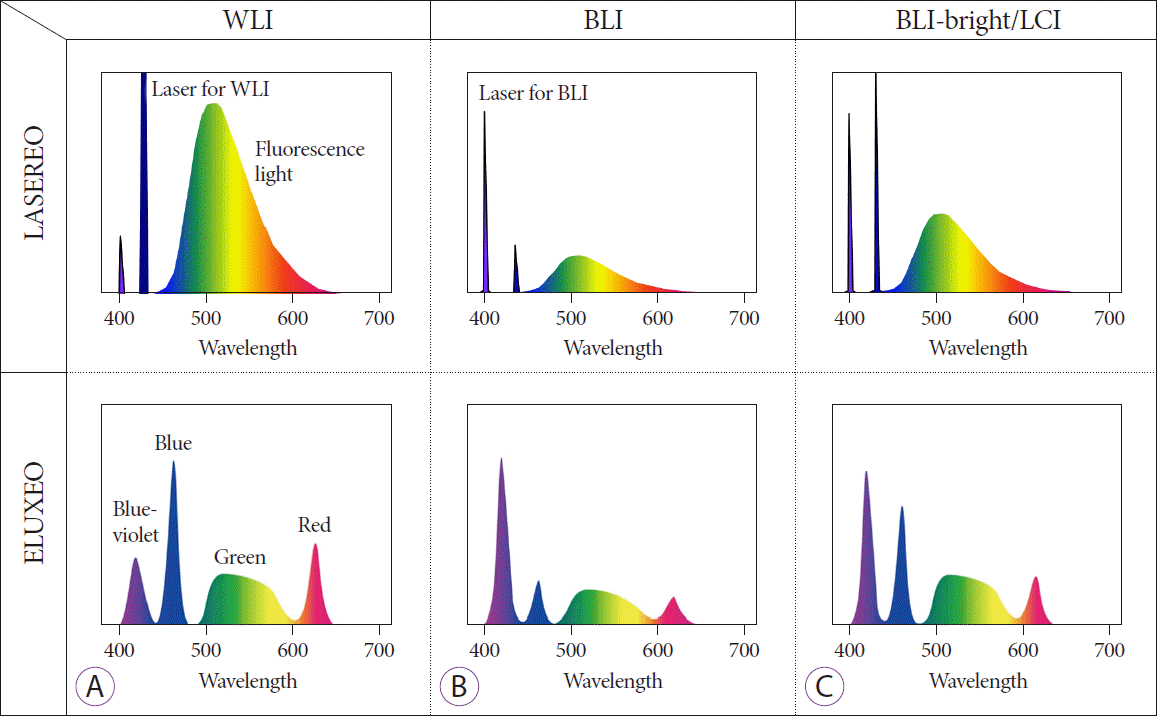
Fig. 2.
Differences in brightness among the three observation modes. (A) Linked color imaging (LCI). (B) White light imaging (WLI). (C) Blue laser/light imaging (BLI).
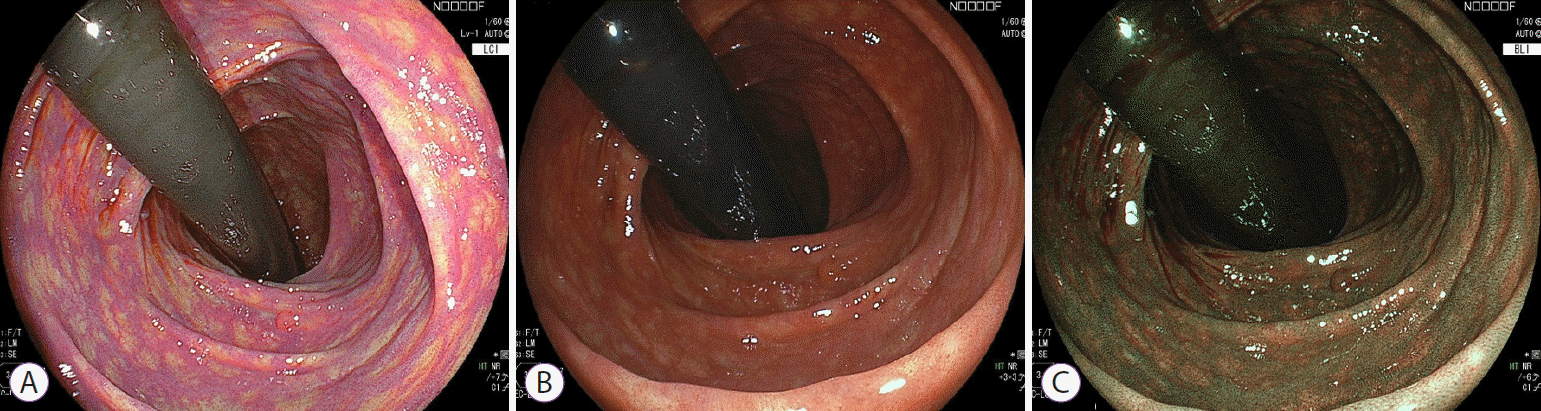
Fig. 3.
An example of the clinical application of the three observation modes. (A, B) White light imaging (WLI) or linked color imaging (LCI) for the detection of polyps. (C) Characterization or depth diagnosis was performed by evaluating the vessels and surface pattern using magnifying blue laser/light imaging (BLI). Irregular vessel and surface patterns are shown in the center of the lesion. (D) Chromoendoscopy with crystal violet staining was additionally performed to confirm the depth diagnosis as a final judgment. A severe irregular pit pattern was found at the center of the lesion. From this finding, the depth diagnosis was a deep submucosal invasion.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download