1. Dulai GS, Jensen DM, Kovacs TOG, Gralnek IM, Jutabha R. Endoscopic treatment outcomes in watermelon stomach patients with and without portal hypertension. Endoscopy. 2004; 36:68–72.

2. Sebastian S, McLoughlin R, Qasim A, O’Morain CA, Buckley MJ. Endoscopic argon plasma coagulation for the treatment of gastric antral vascular ectasia (watermelon stomach): long-term results. Dig Liver Dis. 2004; 36:212–217.

3. Gretz JE, Achem SR. The watermelon stomach: clinical presentation, diagnosis, and treatment. Am J Gastroenterol. 1998; 93:890–895.

4. Ripoll C, Garcia-Tsao G. Management of gastropathy and gastric vascular ectasia in portal hypertension. Clin Liver Dis. 2010; 14:281–295.

5. Fuccio L, Mussetto A, Laterza L, Eusebi LH, Bazzoli F. Diagnosis and management of gastric antral vascular ectasia. World J Gastrointest Endosc. 2013; 5:6–13.

6. Hsu W-H, Wang Y-K, Hsieh M-S, et al. Insights into the management of gastric antral vascular ectasia (watermelon stomach). Therap Adv Gastroenterol. 2018; 11:1756283X17747471.

7. Ito M, Uchida Y, Kamano S, Kawabata H, Nishioka M. Clinical comparisons between two subsets of gastric antral vascular ectasia. Gastrointest Endosc. 2001; 53:764–770.

8. Thomas A, Koch D, Marsteller W, Lewin D, Reuben A. An analysis of the clinical, laboratory, and histological features of striped, punctate, and nodular gastric antral vascular ectasia. Dig Dis Sci. 2018; 63:966–973.

9. Selinger CP, Ang YS. Gastric antral vascular ectasia (GAVE): an update on clinical presentation, pathophysiology and treatment. Digestion. 2008; 77:131–137.

10. Spahr L, Villeneuve JP, Dufresne MP, et al. Gastric antral vascular ectasia in cirrhotic patients: absence of relation with portal hypertension. Gut. 1999; 44:739–742.

11. Tekola BD, Caldwell S. Approach to the management of portal hypertensive gastropathy and gastric antral vascular ectasia. Clin Liver Dis (Hoboken). 2012; 1:163–166.

12. Albitar HAH, Almodallal Y, Papadakis KA, Rajan E, Kamath PS, Iyer VN. Intravenous bevacizumab reduces transfusion requirements and endoscopic interventions in patients with gastric antral vascular ectasia and small bowel angioectasia. Gastroenterology. 2020; 158:1162–1163.e4.

13. Kamath PS, Lacerda M, Ahlquist DA, McKusick MA, Andrews JC, Nagorney DA. Gastric mucosal responses to intrahepatic portosystemic shunting in patients with cirrhosis. Gastroenterology. 2000; 118:905–911.

14. Brito HP, Ribeiro IB, de Moura DTH, et al. Video capsule endoscopy vs double-balloon enteroscopy in the diagnosis of small bowel bleeding: A systematic review and meta-analysis. World J Gastrointest Endosc. 2018; 10:400–421.

15. Ribeiro IB, Rezende DT, Madruga Neto AC, et al. Endoscopic dual therapy for giant peptic ulcer hemorrhage. Endoscopy. 2018; 50:E316–E317.

16. Lôbo MR de A, Chaves DM, DE Moura DTH, Ribeiro IB, Ikari E, DE Moura EGH. Safety and efficacy of EUS-guided coil plus cyanoacrylate versus conventional cyanoacrylate technique in the treatment of gastric varices: a randomized controlled trial. Arq Gastroenterol. 2019; 56:99–105.
17. Luz GO, Matuguma SE, Madruga Neto AC, et al. A novel technique in the management of refractory variceal bleeding. Endoscopy. 2020; 52:310–311.

18. de Rezende DT, Brunaldi VO, Bernardo WM, et al. Use of hemostatic powder in treatment of upper gastrointestinal bleeding: a systematic review and meta-analysis. Endosc Int Open. 2019; 7:E1704–E1713.

19. ASGE technology committee, Parsi MA, Schulman AR, et al. Devices for endoscopic hemostasis of nonvariceal GI bleeding (with videos). VideoGIE. 2019; 4:285–299.

20. Boltin D, Gingold-Belfer R, Lichtenstein L, Levi Z, Niv Y. Long-term treatment outcome of patients with gastric vascular ectasia treated with argon plasma coagulation. Eur J Gastroenterol Hepatol. 2014; 26:588–593.

21. Chaves DM, Sakai P, Oliveira CV, Cheng S, Ishioka S. Watermelon stomach: clinical aspects and treatment with argon plasma coagulation. Arq Gastroenterol. 2006; 43:191–195.

22. Zepeda-Gómez S, Sultanian R, Teshima C, Sandha G, Van Zanten S, Montano-Loza AJ. Gastric antral vascular ectasia: a prospective study of treatment with endoscopic band ligation. Endoscopy. 2015; 47:538–540.

23. Eccles J, Falk V, Montano-Loza AJ, Zepeda-Gómez S. Long-term follow-up in patients with gastric antral vascular ectasia (GAVE) after treatment with endoscopic band ligation (EBL). Endosc Int Open. 2019; 7:E1624–E1629.

24. Swanson E, Mahgoub A, MacDonald R, Shaukat A. Medical and endoscopic therapies for angiodysplasia and gastric antral vascular ectasia: a systematic review. Clin Gastroenterol Hepatol. 2014; 12:571–582.

25. Gross SA, Al-Haddad M, Gill KRS, Schore AN, Wallace MB. Endoscopic mucosal ablation for the treatment of gastric antral vascular ectasia with the HALO90 system: a pilot study. Gastrointest Endosc. 2008; 67:324–327.

26. Dray X, Repici A, Gonzalez P, et al. Radiofrequency ablation for the treatment of gastric antral vascular ectasia. Endoscopy. 2014; 46:963–969.

27. Mondragón OH, Valenzuela LL, Valencia JB, Saavedra DE, Velasco GB. Safety and efficacy of hybrid-APC for the treatment of refractory GAVE. In: Endoscopy [Internet]. Leipzig: Georg Thieme Verlag KG;c2018. [updated 2018 Apr 21; cited 2021 Apr 16]. p. ePP069V. Available from:
http://www.thieme-connect.de/DOI/DOI?10.1055/s-0038-1637393.
28. Nakamura S, Mitsunaga A, Konishi H, Oi I, Shiratori K, Suzuki S. Long-term follow up of gastric antral vascular ectasia treated by argon plasma coagulation. Dig Endosc. 2006; 18:128–133.

29. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097.

30. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 [Internet]. London (UK): c2019. [updated 2019 July; cited 2021 Apr 16]. Available from:
https://training.cochrane.org/handbook.
31. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005; 5:13.

32. Wells CD, Harrison ME, Gurudu SR, et al. Treatment of gastric antral vascular ectasia (watermelon stomach) with endoscopic band ligation. Gastrointest Endosc. 2008; 68:231–236.

33. Sato T, Yamazaki K, Akaike J. Endoscopic band ligation versus argon plasma coagulation for gastric antral vascular ectasia associated with liver diseases. Dig Endosc. 2012; 24:237–242.

34. Wells CD, Harrison ME, Gurudu SR, et al. Treatment of gastric antral vascular ectasia (watermelon stomach) with endoscopic band ligation. Gastrointest Endosc. 2008; 68:231–236.

35. Keohane J, Berro W, Harewood GC, Murray FE, Patchett SE. Band ligation of gastric antral vascular ectasia is a safe and effective endoscopic treatment. Dig Endosc. 2013; 25:392–396.

36. Ghaffar MMA, Maguid HMAE. Endoscopic band ligation versus argon plasma coagulation in management of bleeding from gastric antral vascular ectasia in patients with portal hypertension. Journal of Medicine in Scientific Research. 2019; 2:214–219.
37. Abdelhalim H, Mostafa I, Abdelbary MS, Elansary M, Abdo M, Rahim AA. Endoscopic band ligation versus argon plasma coagulation for the treatment of gastric antral vascular ectasia in Egyptian patients with liver cirrhosis. World J Med Sci. 2014; 10:357–361.
38. Elhendawy M, Mosaad S, Alkhalawany W, et al. Randomized controlled study of endoscopic band ligation and argon plasma coagulation in the treatment of gastric antral and fundal vascular ectasia. United European Gastroenterol J. 2016; 4:423–428.

39. Ghobrial C, Rabea M, Mohsen N, Eskander A. Gastric antral vascular ectasia in portal hypertensive children: Endoscopic band ligation versus argon plasma coagulation. J Pediatr Surg. 2019; 54:1691–1695.

40. Amer K, Abd El Wahab N, Ibrahim A. Argon plasma coagulation versus endoscopic band ligation in treatment of gastric antral vascular ectasia in cirrhotic patients in Zagazig University Hospitals. Afro-Egyptian Journal of Infectious and Endemic Diseases. 2019; 9:176–184.
41. Chalhoub JM, Umar J, Groudan K, Hamadeh N, Desilets DJ, Greeff Y. Endoscopic band ligation compared to thermal therapy for gastric antral vascular ectasia: a systematic review and meta-analysis. United European Gastroenterol J. 2020; 2050640620975243.

42. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010; 71:446–454.

43. Farooq FT, Wong RCK, Yang P, Post AB. Gastric outlet obstruction as a complication of argon plasma coagulation for watermelon stomach. Gastrointest Endosc. 2007; 65:1090–1092.

44. Izquierdo S, Rey E, Gutiérrez Del Olmo A, Almansa C, Andrés Ramírez Armengol J, Díaz-Rubio M. Polyp as a complication of argon plasma coagulation in watermelon stomach. Endoscopy. 2005; 37:921.

45. Baudet JS, Salata H, Soler M, et al. Hyperplastic gastric polyps after argon plasma coagulation treatment of gastric antral vascular ectasia (GAVE). Endoscopy. 2007; 39 Suppl 1:E320.

46. Quevedo R, Moehlen M, Joshi V. Gastric inflammatory polyps as a sequela of radiofrequency ablation for the treatment of gastric antral vascular ectasia: 1006. ACG. 2011; 106:S375.
47. Tantau M, Crisan D. Is endoscopic band ligation the gold standard for gastric antral vascular ectasia? Endosc Int Open. 2019; 7:E1630–E1631.

48. Burak KW, Lee SS, Beck PL. Portal hypertensive gastropathy and gastric antral vascular ectasia (GAVE) syndrome. Gut. 2001; 49:866–872.

49. Vincent C, Pomier-Layrargues G, Dagenais M, et al. Cure of gastric antral vascular ectasia by liver transplantation despite persistent portal hypertension: a clue for pathogenesis. Liver Transpl. 2002; 8:717–720.

50. Schulz SW, O’Brien M, Maqsood M, Sandorfi N, Del Galdo F, Jimenez SA. Improvement of severe systemic sclerosis-associated gastric antral vascular ectasia following immunosuppressive treatment with intravenous cyclophosphamide. J Rheumatol. 2009; 36:1653–1656.

51. Sepanlou SG, Safiri S, Bisignano C, et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020; 5:245–266.
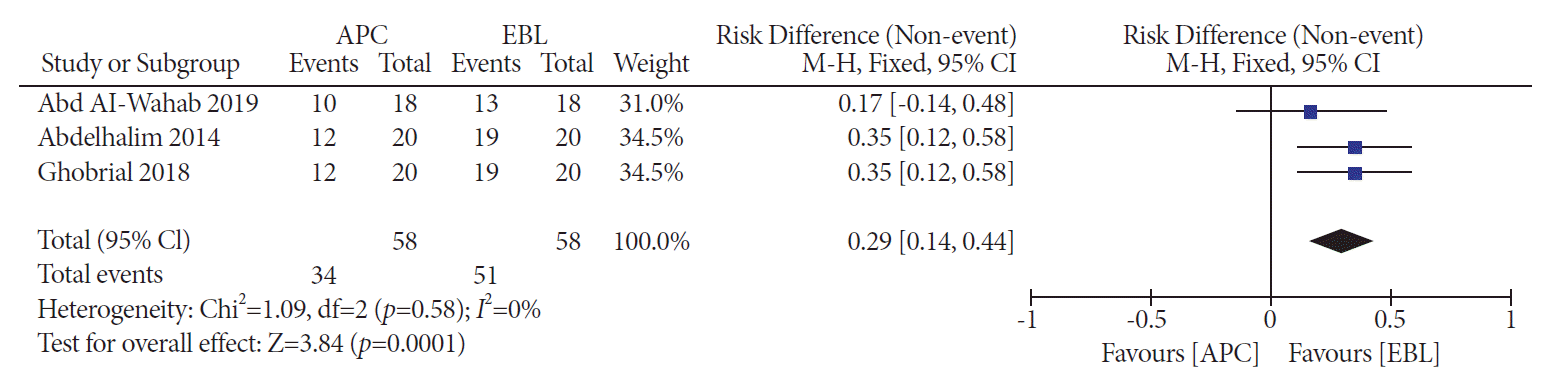
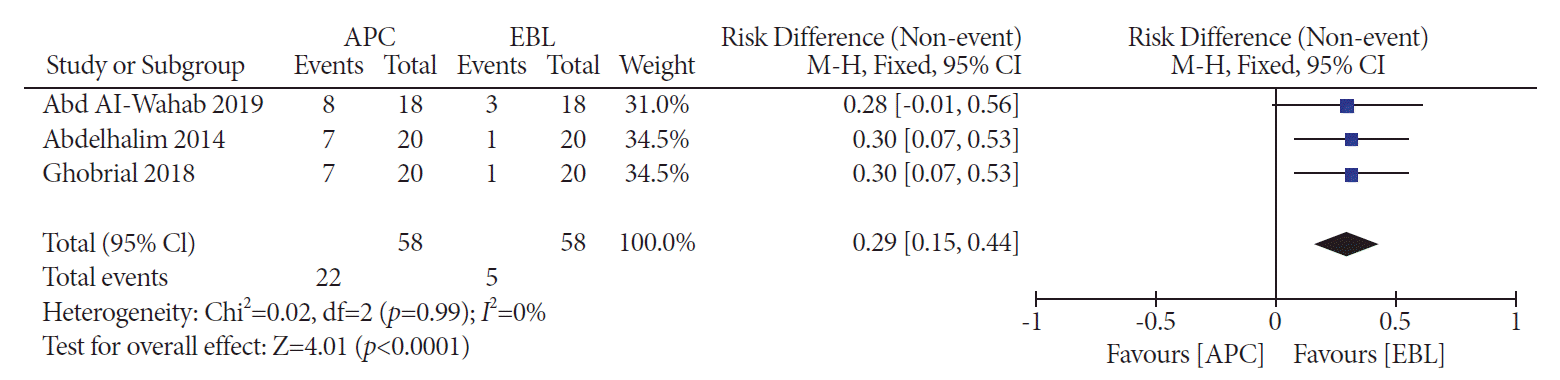
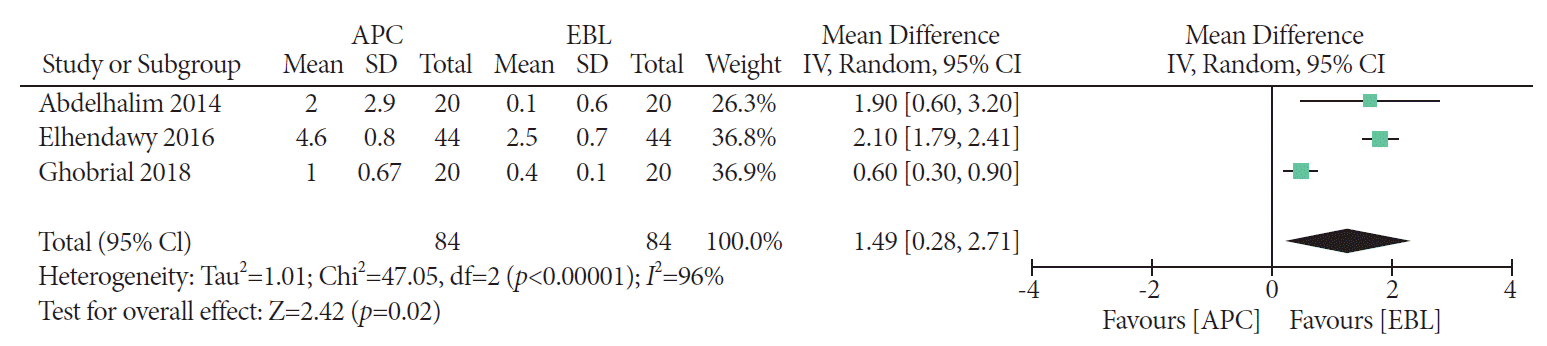
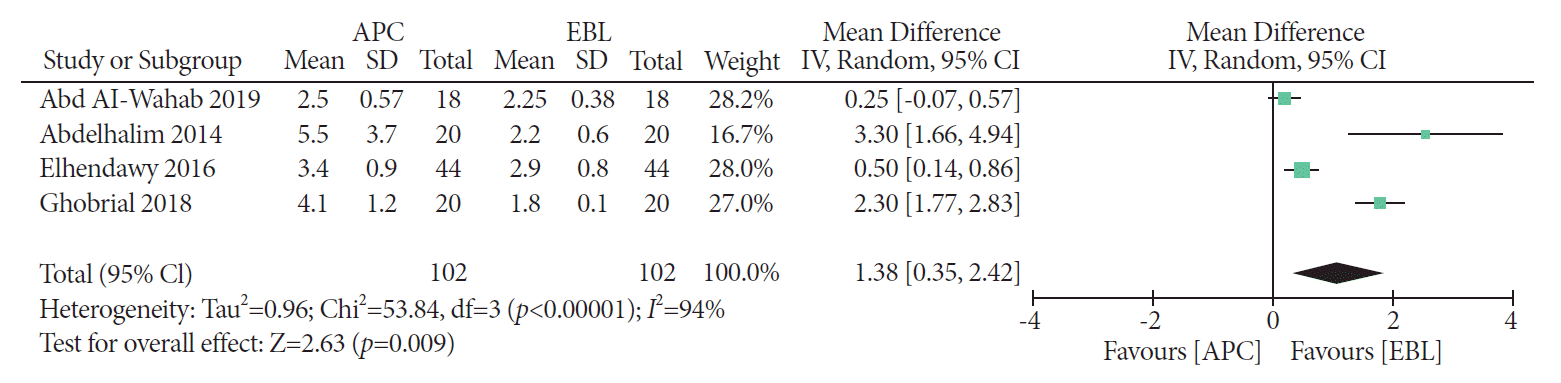




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download