This article has been
cited by other articles in ScienceCentral.
Abstract
Background/Aims
Endoscopic transpapillary gallbladder drainage (ETPGBD) is gaining popularity for the management of acute cholecystitis (AC) in high-risk patients. However, the stents placed during the procedure are not immune to obstruction. Here we describe a novel technique of stenting with two transpapillary stents and evaluate its technical feasibility, safety, and efficacy in AC.
Methods
A retrospective analysis of all patients undergoing ETPGBD using dual stents for AC at our institution between November 1, 2017 and August 31, 2020 was conducted. We abstracted patient data to evaluate technical and clinical success, adverse events, and long-term outcomes. Two stents were placed either during the index procedure or during an interval procedure performed 4–6 weeks after the index procedure.
Results
A total of 21 patients underwent ETPGBD with dual stenting (57.14% male, mean age: 62.14±17.21 years). The median interval between the placement of the first and the second stents was 37 days (range: 0–226 days). Technical and clinical success rates were 100%, with a recurrence rate of 4.76% (n=1) and adverse event rate of 9.52% (n=2) during a mean follow-up period of 471.74±345.64 days (median: 341 days, range: 55–1084 days).
Conclusions
ETPGBD with dual gallbladder stenting is a safe and effective technique for long-term gallbladder drainage in non-surgical candidates. Larger controlled studies are needed to validate our findings for the widespread implementation of this technique.
Go to :

Keywords: Acute cholecystitis, Endoscopic cholecystostomy, Gallbladder drainage
INTRODUCTION
Selective cannulation of the cystic duct during endoscopic retrograde cholangiopancreatography (ERCP) for the retrieval of bile for analysis, culture, and sensitivity and potential gallstone dissolution was first described by Kozarek in 1984 [
1]. This paved the way for endoscopic therapy of acute cholecystitis (AC), with Tamada et al. and Siegel et al. describing their case series of endoscopic transpapillary gallbladder drainage (ETPGBD) in the early 1990s [
2,
3]. Given their low success rates and the mainstream adaptation of laparoscopic cholecystectomies, this approach fell out of favor. Recently, there has been renewed interest in endoscopic drainage techniques including ETPGBD and endoscopic ultrasound-guided gall bladder drainage (EUS-GBD) for high-risk surgical patients with AC [
4,
5]. The advantage of endoscopic techniques over the more traditional approach of percutaneous gallbladder drainage (PGBD) in this population is that in addition to providing immediate drainage and control of acute cholecystitis, these techniques have the advantage of preventing recurrence in the long-term [
4,
5].
The concern for stent obstruction and recurrent cholecystitis has been one of the major issues with ETPGBD due to the small diameter of cystic duct stents, especially when placed on a long-term basis. Multiple strategies have been described to reduce the rate of recurrence, including routine replacement and upsizing of stents to allow for continued drainage [
6,
7]. We used a novel alternative approach with the placement of two gallbladder stents (dual stenting) to potentially avoid occlusion and recurrence. The aim of this study was to evaluate the technical feasibility, efficacy, and safety of this technique.
Go to :

MATERIALS AND METHODS
A retrospective analysis of a prospectively collected database of all patients undergoing ETPGBD for AC using dual stents at our facility between November 1, 2017 and August 31, 2020. We abstracted and analyzed patient demographics, endoscopic imaging, clinical management, and follow-up data to evaluate the technical and clinical success, adverse events, and long-term outcomes. Technical success was defined as successful placement of dual transpapillary gallbladder stents during the index procedure or interval procedure. Clinical success was defined as clinical resolution of AC after the index procedure. The study was approved by our institutional review board (study number 19-574). Descriptive analysis was performed with mean and standard deviation used to describe parametric (normal) data, and median and range used for non-parametric (non-normal) data.
Procedure
A single high-volume (>750 ERCPs/year) therapeutic pancreatobiliary endoscopist with significant experience and expertise in gallbladder stenting performed all procedures. All patients were evaluated by our General Surgery service and deemed high-risk operative candidates for cholecystectomy prior to the endoscopic procedure. ERCP with biliary sphincterotomy and extraction balloon sweeps of the bile duct were performed in a standard fashion. Subsequently, the cystic duct was selectively cannulated using a 0.035-inch angled guidewire (GLIDEWIRE
®; Terumo Medical Corporation, Somerset, NJ, USA). Additional guidewires (0.025-inch angled guidewire [Visiglide
®; Olympus Medical Systems, Tokyo, Japan] and 0.018-inch angled gold-tipped Terumo guidewire [GLIDEWIRE
®; Terumo Medical Corporation, Somerset, NJ, USA]), catheters (rotatable Truetome sphincterotome [TRUEtome
TM; Boston Scientific, Marlborough, MA, USA] and 3–4–5 Fr ultra-tapered tip catheter [Contour
TM ERCP Cannula; Boston Scientific, Marlborough, MA, USA]), and 9–12 mm Extractor Pro XL stone extraction balloon (Extractor
TM Pro; Boston Scientific, Marlborough, MA, USA) were used as needed to achieve successful cannulation in difficult cases. The wire was carefully navigated through the cystic duct, advanced into the gallbladder, and allowed to coil twice within the gallbladder lumen under fluoroscopic guidance. Subsequently, a transpapillary (6 Fr × 22 cm or 7 Fr × 22 cm) double-pigtail soft plastic stent (Polaris Medical Inc.; Burlington, ON, Canada) was placed across the bile duct and the cystic duct into the gallbladder, with the proximal end of the pigtail in the gallbladder lumen and distal end in the duodenal lumen. A second stent was placed either during the index procedure or an interval procedure 4–6 weeks after the index procedure, based on the discretion of the endoscopist (
Fig. 1).
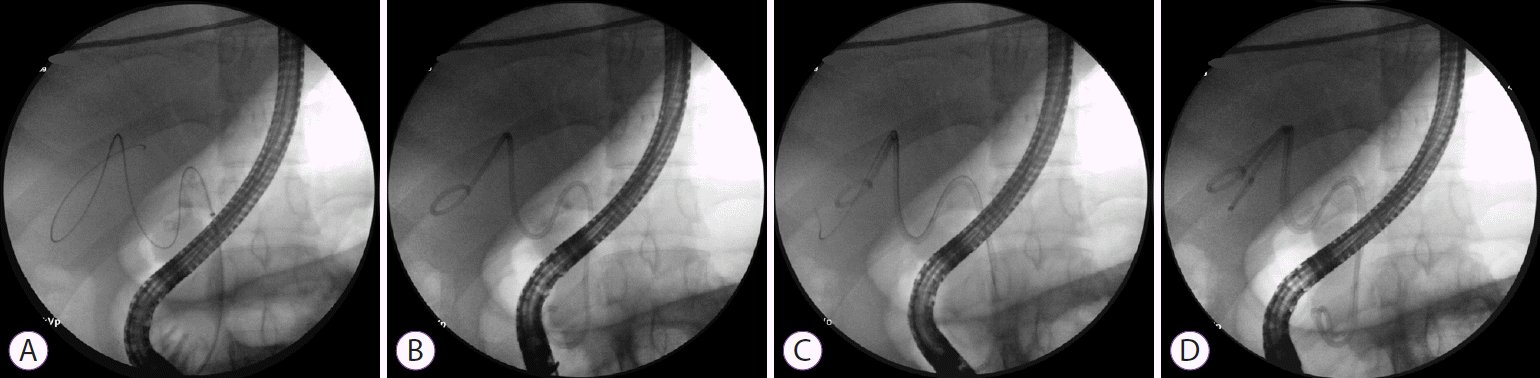 | Fig. 1.Fluoroscopic images show a guidewire coiled within the gallbladder (A) followed by placement of the first transpapillary gallbladder stent (B) During the interval endoscopic retrograde cholangiopancreatography, selective cystic duct cannulation was achieved along the existing stent and the guidewire was advanced again into the gallbladder (C) followed by placement of the second transpapillary gallbladder stent (dual stenting) (D) for long-term gallbladder drainage in a non-surgical patient with acute cholecystitis. 
|
Go to :

RESULTS
A total of 21 patients underwent dual transpapillary gallbladder stenting for AC. The mean age of the patients was 62.14±17.21 years and 60% (n=12) of the patients were male. A total of 43 stents were placed; of which 67.44% (29/43) were 7 Fr in size, 30.23% (13/43) were 6 Fr in size, and 2.33% (1/43) was 8.5 Fr in size. An extra stent was used in one patient, asthe patient had inadvertent removal of the existing transpapillary gallbladder stent during removal of the external percutaneous transhepatic cholecystostomy tube prior to the second procedure. Only 1 patient (4.76%) had both stents placed during the index procedure. The median interval between the placement of the first and second stent was 39 days (range: 0–226 days). The first and the second gallbladder stents were successfully placed in all 21 patients who underwent ETPGBD, with technical and clinical success rates of 100%.
The patients were followed for a mean duration of 471.74±345.64 days (median: 341 days, range: 55–1084 days). Two patients (9.52%) were lost to follow-up after the second procedure. One patient underwent cholecystectomy after an interval of 40 days. Recurrent cholecystitis occurred in one (4.76%) patient 315 days after the index procedure and was managed by EUS-GBD using a lumen-apposing metal stent (LAMS). Two patients (9.52%) experienced adverse events during this period. These included incidental finding of stent migration in one patient (one of the two stents was not visualized on abdominal computed tomography performed for an unrelated reason) and delayed post-sphincterotomy bleeding in one patient, which was managed with ERCP and placement of a fully covered metal biliary stent. Severity per the American Society for Gastrointestinal Endoscopy Lexicon of Adverse Events was mild (4.76%, n=1) and moderate (4.76%, n=1), respectively.
Go to :

DISCUSSION
Laparoscopic cholecystectomy is the mainstay in the management of AC. In high-risk patients, PGBD has been the standard approach [
8]. Recently, there has been renewed interest in endoscopic management of AC in high-risk and/or non-surgical candidates. The primary driving force for endoscopic therapy is that in addition to allowing adequate gallbladder drainage in the acute phase, these modalities have the ability to mitigate future episodes and long-term recurrence of cholecystitis [
5]. Thus, they have a clear advantage over PGBD, which is by design a temporizing measure that allows gallbladder drainage until inflammation resolves.
In our practice, ETPGBD is preferred over EUS-GBD in patients who may potentially become surgical candidates in the future, as it allows for preservation of the native anatomy. It is also preferred in patients who need simultaneous ERCP for other indications such as choledocholithiasis. Further ETPGBD is significantly more cost-effective than EUS-GBD, considering the additional cost of cautery-enhanced LAMS (CE-LAMS). However, a recent meta-analysis including 857 patients compared EUS-GBD and ETPGBD and found higher technical (pooled odds ratio [OR], 5.22;
p=0.0006,
I2=20%) and clinical success (pooled OR, 4.16;
p=0.0001,
I2=19%) and lower recurrence (pooled OR, 0.33;
p=0.01;
I2=0%) in patients undergoing EUS-GBD when compared to ETPGBD [
9]. Smaller studies have reported recurrence rates as high as 18.8% in patients undergoing ETPGBD compared to 2.6% in patients undergoing EUS-GBD [
10].
This increased recurrence rate is mostly attributed to the relative difference in size between the stents used for the procedures (7 Fr, ~2.3 mm vs. 6–10 mm, respectively). The smaller diameter of the plastic stents used for ETPGBD makes them prone to obstruction and recurrence. In addition, the dumbbell-shaped design of CE-LAMS prevents migration, offering sustained drainage of the gallbladder.
In order to address this issue, certain studies had initially advocated for routine replacement with potential upsizing of stents [
6,
7]. Here we describe a novel strategy to prevent stent obstruction and recurrence using dual stenting. This strategy was adopted based on effectiveness and safety data from the placement of multiple plastic stents for endoscopic management of benign biliary strictures [
11-
13]. Hypothetically, dual stents have lower chances of occlusion. However, in the event both stents get occluded, the increased surface area along the dual stents allows enhanced capillary action along the outer surface of the stents (“wicking” effect), making patency non-essential [
14,
15]. Moreover, placement of dual stents may also result in dilation of the cystic duct.
We followed the patients for a mean duration of 15.7 months. During this period, we observed only one case of recurrent cholecystitis at 315 days after the index procedure, which was managed with EUS-GBD. It is difficult to hypothesize if the low recurrence rate observed in our study during a maximum follow-up period of 1,084 days can be attributed to enhanced luminal patency or wicking across the surface of the stents. However, this difference is mainly academic curiosity, as the clinical outcome is not dependent on the underlying mechanism.
Placement of both the stents during the index procedure is advantageous, as it avoids a second ERCP and the associated risks and costs. However, this is not always possible and is prone to a higher risk of adverse events due to active inflammation and edema of the cystic duct and gallbladder that predisposes these structures to injury and perforation while attempting to pass guidewires and stents. Hence, in most of the patients, we placed only one stent during the index procedure to allow drainage and resolution of acute inflammation. This was followed by an interval procedure a few weeks later when the inflammation had resolved to place the second stent for long-term drainage and prevention of recurrence.
Our study is the first one to evaluate the technical feasibility, efficacy, and safety of a novel dual gallbladder stenting technique. However, it has several limitations. Although we have a prospective database, the study was a retrospective analysis with the inherent limitations of a retrospective case series with heterogeneity in patient population. Additionally, this was a single-center, single-operator study wherein all procedures were performed by a high-volume endoscopist with extensive experience and expertise in performing ETPGBD with a high success rate, which limits the generalizability of our results. Therefore, the results of our study may not be applicable to lower-volume centers with less experience in complex ERCP techniques. Further the high technical success rate is likely an overestimation due to a relatively small sample size and selection bias. Lastly, long-term clinical outcomes and cost-effectiveness of this technique need to be evaluated in future studies.
In conclusion, ETPGBD with dual gallbladder stenting is an effective and safe technique for long-term gallbladder drainage in non-surgical candidates with acute cholecystitis. Further this does not alter native anatomy which is especially important in patients who may become surgical candidates in the future. Larger controlled studies are needed to validate our findings for the widespread implementation of this technique.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download