1. Park DH, Jang JW, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with transluminal stenting after failed ERCP: predictors of adverse events and long-term results. Gastrointest Endosc. 2011; 74:1276–1284.

2. Vila JJ, Pérez-Miranda M, Vazquez-Sequeiros E, et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: a Spanish national survey. Gastrointest Endosc. 2012; 76:1133–1141.

3. Poincloux L, Rouquette O, Buc E, et al. Endoscopic ultrasound-guided biliary drainage after failed ERCP: cumulative experience of 101 procedures at a single center. Endoscopy. 2015; 47:794–801.

4. Khashab MA, Messallam AA, Penas I, et al. International multicenter comparative trial of transluminal EUS-guided biliary drainage via hepatogastrostomy vs. choledochoduodenostomy approaches. Endosc Int Open. 2016; 4:E175–E181.

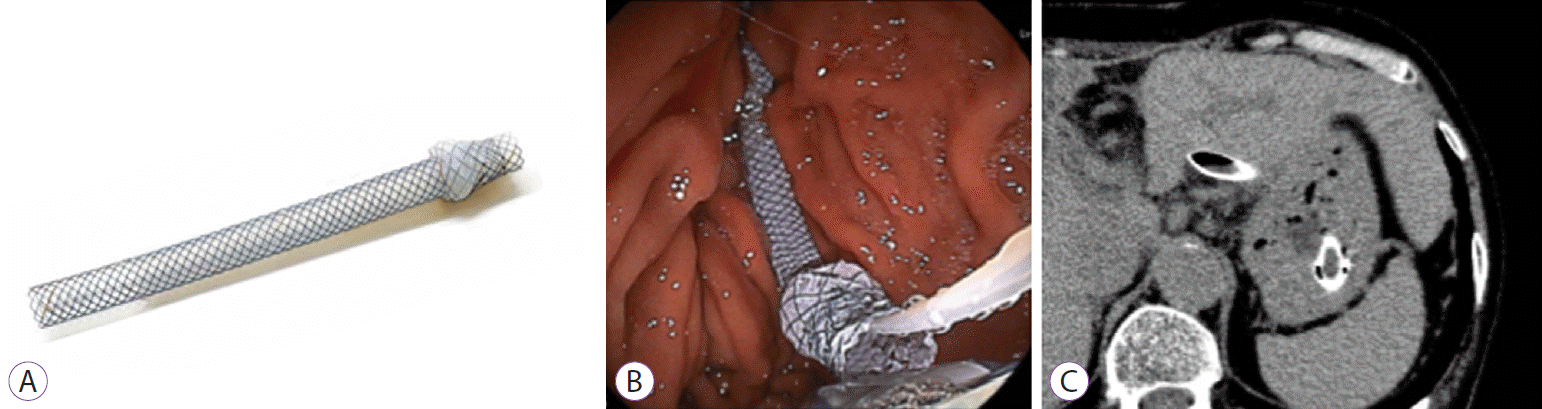
5. Nakai Y, Isayama H, Yamamoto N, et al. Safety and effectiveness of a long, partially covered metal stent for endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction. Endoscopy. 2016; 48:1125–1128.

6. Minaga K, Takenaka M, Kitano M, et al. Rescue EUS-guided intrahepatic biliary drainage for malignant hilar biliary stricture after failed transpapillary re-intervention. Surg Endosc. 2017; 31:4764–4772.

7. Sportes A, Camus M, Greget M, et al. Endoscopic ultrasound-guided hepaticogastrostomy versus percutaneous transhepatic drainage for malignant biliary obstruction after failed endoscopic retrograde cholangiopancreatography: a retrospective expertise-based study from two centers. Therap Adv Gastroenterol. 2017; 10:483–493.

8. Oh D, Park DH, Song TJ, et al. Optimal biliary access point and learning curve for endoscopic ultrasound-guided hepaticogastrostomy with transmural stenting. Therap Adv Gastroenterol. 2017; 10:42–53.

9. Honjo M, Itoi T, Tsuchiya T, et al. Safety and efficacy of ultra-tapered mechanical dilator for EUS-guided hepaticogastrostomy and pancreatic duct drainage compared with electrocautery dilator (with video). Endosc Ultrasound. 2018; 7:376–382.

10. Paik WH, Lee TH, Park DH, et al. EUS-guided biliary drainage versus ERCP for the primary palliation of malignant biliary obstruction: a multicenter randomized clinical trial. Am J Gastroenterol. 2018; 113:987–997.

11. Nakai Y, Sato T, Hakuta R, et al. Long-term outcomes of a long, partially covered metal stent for EUS-guided hepaticogastrostomy in patients with malignant biliary obstruction (with video). Gastrointest Endosc. 2020; 92:623–631.e1.

12. Krishnamoorthi R, Dasari CS, Thoguluva Chandrasekar V, et al. Effectiveness and safety of EUS-guided choledochoduodenostomy using lumen-apposing metal stents (LAMS): a systematic review and meta-analysis. Surg Endosc. 2020; 34:2866–2877.

13. Ogura T, Nishioka N, Ueno S, et al. Effect of echoendoscope angle on success of guidewire manipulation during endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy. 2021; 53:369–375.

14. Ma KW, So H, Cho DH, Oh JS, Cheung TT, Park DH. Durability and outcome of endoscopic ultrasound-guided hepaticoduodenostomy using a fully covered metal stent for segregated right intrahepatic duct dilatation. J Gastroenterol Hepatol. 2020; 35:1753–1760.

15. Ogura T, Kitano M, Takenaka M, et al. Multicenter prospective evaluation study of endoscopic ultrasound-guided hepaticogastrostomy combined with antegrade stenting (with video). Dig Endosc. 2018; 30:252–259.

16. Amano M, Ogura T, Onda S, et al. Prospective clinical study of endoscopic ultrasound-guided biliary drainage using novel balloon catheter (with video). J Gastroenterol Hepatol. 2017; 32:716–720.

17. Ogura T, Nakai Y, Iwashita T, Higuchi K, Itoi T. Novel fine gauge electrocautery dilator for endoscopic ultrasound-guided biliary drainage: experimental and clinical evaluation study (with video). Endosc Int Open. 2019; 7:E1652–E1657.

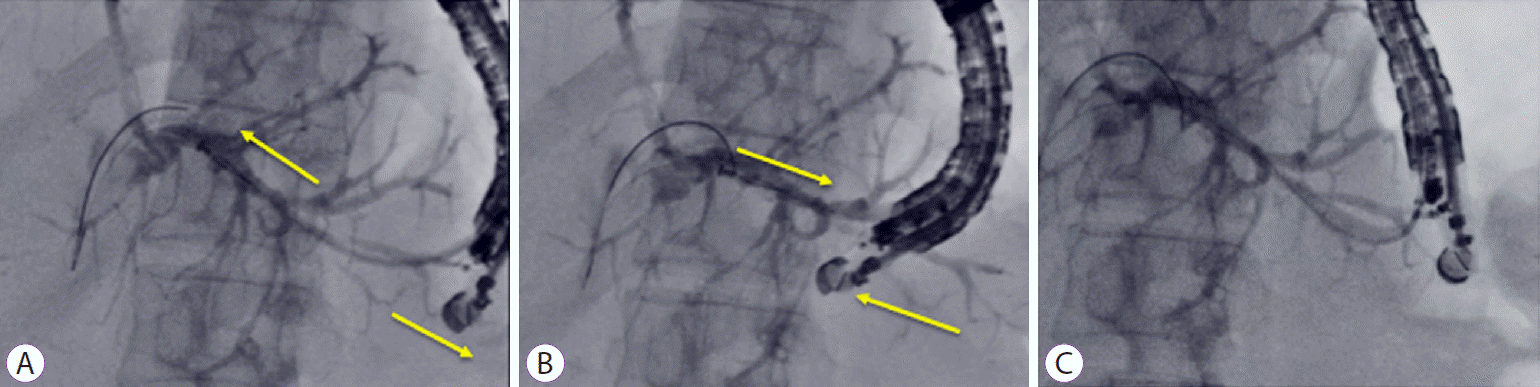
18. Ogura T, Masuda D, Takeuchi T, Fukunishi S, Higuchi K. Liver impaction technique to prevent shearing of the guidewire during endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy. 2015; 47:E583–E584.

19. Kwon CI, Koh DH, Song TJ, Park WS, Lee DH, Jeong S. Technical reports of endoscopic retrograde cholangiopancreatography guidewires on the basis of physical properties. Clin Endosc. 2020; 53:65–72.

20. Ryou M, Benias PC, Kumbhari V. Initial clinical experience of a steerable access device for EUS-guided biliary drainage. Gastrointest Endosc. 2020; 91:178–184.

21. Miyano A, Ogura T, Yamamoto K, Okuda A, Nishioka N, Higuchi K. Clinical impact of the intra-scope channel stent release technique in preventing stent migration during EUS-guided hepaticogastrostomy. J Gastrointest Surg. 2018; 22:1312–1318.

22. Cho DH, Lee SS, Oh D, et al. Long-term outcomes of a newly developed hybrid metal stent for EUS-guided biliary drainage (with videos). Gastrointest Endosc. 2017; 85:1067–1075.

23. Maehara K, Hijioka S, Sakamoto T, et al. Novel biliary drainage of a choledochojejunal anastomotic stenosis using a double-balloon endoscope and forward-viewing endoscopic ultrasound. Endoscopy. 2021; 53:E242–E244.

24. Ogura T, Yamada M, Nishioka N, Yamada T, Higuchi K. One-step stent deployment of EUS-guided hepaticogastrostomy using a novel covered metal stent with a fine-gauge stent delivery system (with video). Endosc Ultrasound. 2020; 9:267–269.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download