Abstract
Background/Aims
Recent studies have reported the favorable outcomes of underwater endoscopic mucosal resection (UEMR) for colorectal polyps. We performed a systematic review and meta-analysis evaluating the efficacy and safety of UEMR for nonpedunculated polyps ≥10 mm.
Methods
We performed a comprehensive search of multiple databases (through May 2020) to identify studies reporting the outcomes of UEMR for ≥10 mm nonpedunculated colorectal polyps. The assessed outcomes were recurrence rate on the first follow-up, en bloc resection, incomplete resection, and adverse events after UEMR.
Results
A total of 1276 polyps from 16 articles were included in our study. The recurrence rate was 7.3% (95% confidence interval [CI], 4.3–12) and 5.9% (95% CI, 3.6–9.4) for nonpedunculated polyps ≥10 and ≥20 mm, respectively. For nonpedunculated polyps ≥10 mm, the en bloc resection, R0 resection, and incomplete resection rates were 57.7% (95% CI, 42.4–71.6), 58.9% (95% CI, 42.4–73.6), and 1.5% (95% CI, 0.8–2.6), respectively. The rates of pooled adverse events, intraprocedural bleeding, and delayed bleeding were 7.0%, 5.4%, and 2.9%, respectively. The rate of perforation and postpolypectomy syndrome was 0.8%.
Colorectal cancer is the fourth most common malignancy and represents 8.2% of all new cancer cases in the United States [1]. Various endoscopic techniques depending on the size and location of polyps are available for polyp removal. Endoscopic mucosal resection (EMR) for the removal of large and/or sessile colorectal polyps can be performed with or without water assistance [2,3]. Conventional EMR (CEMR) is performed through submucosal injection to lift the lesion followed by hot snare polypectomy [4]. The US Multi-Society Task Force on Colorectal Cancer and the European Society of Gastrointestinal Endoscopy recommend CEMR for the removal of flat or sessile polyps ≥10 mm in size [5,6]. The clinically significant bleeding and perforation rates after CEMR range from 6% to 15% and from 1% to 2%, respectively [7-9]. CEMR has also been shown to be more cost-effective than surgical resection and endoscopic submucosal dissection (ESD) [10-12]. However, the major argument against CEMR comes from the high rates of residual and/or recurrent polyps, ranging from 16% to 55% [10,13,14]. ESD is considered the treatment of choice for the removal of large lesions in Japan; however, its use has been largely hindered by the limited expertise and high complication rates in the Western world [15].
Binmoeller et al. first described and developed underwater endoscopic mucosal resection (UEMR) in 2012 [16], based on observations during endoscopic ultrasound. In UEMR, the mucosal surface tends to involute inwards and assumes a collapsed state. This obviates the need for submucosal injection, potentially leading to fewer adverse events. Since the first description in 2012, several studies have reported the encouraging outcomes of UEMR for the removal of colorectal polyps [16-20]; however, its utility in large-sized (≥10 and ≥20 mm) nonpedunculated polyps has not been clearly described. A previously published systematic review was limited by the inclusion of patients with submucosal injection and fewer studies [21]. In addition, it included studies reporting outcomes exclusively on resection of recurrent lesions [22,23] and polyps located at the appendiceal orifice. Therefore, we performed a systematic review and meta-analysis to assess the safety and efficacy of UEMR for the removal of nonpedunculated colorectal polyps ≥10 mm.
We conducted a comprehensive search of several databases from inception through May 2020. The databases included MEDLINE® and Epub Ahead of Print, Embase, Ovid Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Scopus. An experienced medical librarian helped with the literature search using inputs from the study authors. A controlled vocabulary supplemented with keywords was used to search for studies of interest. We used the PICO (Participant [adult patients aged ≥18 years with nonpedunculated polyps ≥10 mm], Intervention [UEMR], Comparator [open], Outcomes [effectiveness and safety]) strategy to conduct this meta-analysis. The full search strategy is available in Appendix 1. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and Meta-analysis of Observational Studies in Epidemiology (MOOSE) checklists were followed and are provided in Appendices 2 and 3 [24,25].
We included studies that reported the outcomes of UEMR for the removal of nonpedunculated polyps ≥10 mm. Fulltext articles were included irrespective of the study sample size, inpatient/outpatient setting, and geography, as long as they provided any data needed for the analysis. Nonpedunculated polyps were defined according to the Paris classification of colorectal polyps.
Studies conducted in the pediatric population (age <18 years), conference abstracts, case reports, and studies not published in the English language were excluded. We also excluded studies that reported outcomes of UEMR for recurrent lesions and studies exclusively reporting the outcomes of polyps at specific locations. In cases of multiple publications from the same cohort and/or overlapping cohorts, data from the most recent and/or most appropriate comprehensive report were retained.
Data on study-related outcomes in the individual studies were abstracted onto a standardized form by at least two authors (Rajat Garg, Manik Aggarwal, or Jaideep Bhalla), and two authors (RG, JB, or MA) independently performed the quality scoring. We contacted the primary study authors via email, as needed, for further information and/or clarification on data.
The Newcastle-Ottawascale was used to assess the quality of cohort studies, and the Jadad score was used to assess the quality of randomized controlled trials [26,27]. The details of scores and quality are provided in Supplementary Table 1.
The primary outcome was the pooled rate of residual/recurrent polyps on the first follow-up colonoscopy after the index UEMR at the site of the previous intervention. Recurrent/residual polyps were described on endoscopic and histologic assessments by the study authors.
The pooled rates of en bloc resection, incomplete macroscopic resection, and R0 resection were other outcomes reported by the individual study authors. En bloc resection was defined as the removal of lesions in one piece rather than in multiple small pieces. Incomplete resection was defined as the presence of macroscopic residual polyps based on the endoscopist’s assessment, as reported by the study authors. R0 resection was defined as margins clear of any abnormal tissue on histologic evaluation.
The pooled rate of all adverse events after UEMR was also assessed as an outcome. Adverse events were further categorized into intraprocedural bleeding, delayed bleeding, perforation, and postpolypectomy syndrome (PPS). Intraprocedural bleeding was defined as bleeding requiring endoscopic hemostasis during the procedure, and delayed bleeding was defined as postprocedural bleeding within 2–4 weeks of resection. Delayed bleeding was defined as any postprocedural bleeding that needed emergency department visit, hospitalization, transfusion, or reintervention (endoscopy, angiography, or surgery).
We used meta-analysis techniques to calculate the pooled estimates in each case following the methods suggested by DerSimonian and Laird using the random-effects model [28]. When the incidence of an outcome was zero in a study, a continuity correction of 0.5 was added to the number of incident cases before statistical analysis [29]. Heterogeneity was assessed between study-specific estimates by using the Cochran Q statistical test for heterogeneity [30-32] and the I2 statistics [33,34]. I2 values of <30%, 30%–60%, 61%–75%, and >75% were suggestive of low, moderate, substantial, and considerable heterogeneity, respectively [35]. Publication bias was qualitatively assessed by visual inspection of funnel plots and quantitatively assessed using the Egger test [36]. When publication bias was present, further statistics using Duval and Tweedie’s “trim-and-fill” test were used to ascertain the impact of the bias [37]. Three levels of impact were reported according to the concordance between the reported results and the actual estimate if there were no bias. The impact was reported as minimal if both versions were estimated to be the same, modest if the effect size substantially changed but the final finding remained the same, and severe if the basic final conclusion of the analysis was threatened by the bias [38]. A p-value of ≥0.05 was a-priori used to define statistical significance.
For recurrence rate, adverse events, and intraprocedural bleeding, further meta-regression analyses based on proximal location, mean polyp size, mean age, and study type (prospective or retrospective) was performed to ascertain heterogeneity and predictors. We also performed subgroup analysis to determine the outcomes of UEMR for nonpedunculated polyps ≥20 mm.
All analyses were performed using R statistical software (metafor package; The R Foundation for Statistical Computing, Vienna, Austria).
From the initial 144 studies, 81 records were screened and 20 full-length articles were reviewed. Sixteen studies that reported the outcomes of UEMR for the removal of nonpedunculated polyps ≥10 mm were included in the final analysis [16-18,20,39-50]. Two studies, one reporting outcomes of recurrent lesions [51] and the other exclusively reporting the outcomes of lesions located at the appendiceal orifice, were excluded [52]. The study by Kawamura et al. was also excluded because it reported outcomes for both pedunculated and nonpedunculated polyps, and did not meet the inclusion criteria [53]. We also excluded 29 polyps from the study by Siau et al. that had submucosal injection before resection and 180 polyps from the study by Yen et al. because of their smaller size (6–9 mm) [47,50]. The schematic diagram of study selection is illustrated in Fig. 1.
A total of 1,276 nonpedunculated polyps ≥10 mm removed using UEMR were included in our study. The mean age of patients was 64.9±4.3 years (range 54.5–75 years). The mean polyp size was 20.3±6.9 mm (range 10–38 mm). A total of 852 (66.7%) polyps were proximally located. Proximal polyps were defined as polyps located proximal to the splenic flexure, as described in previous studies. The mean resection time was reported as 15.3±10.5 min (range 3.8–38 min) from nine studies. The population characteristics are described in Table 1, and data on the assessed outcomes are shown in Table 2. Histopathologic data were available in 11 studies, and 5 studies did not provide data on the pathology of nonpedunculated polyps >10 mm. Among the 745 polyps from 11 studies, the most common lesion was adenoma (59.9%, n=446), followed by sessile serrated lesion (17.0%, n=127), high-grade dysplasia (14.8%, n=110), adenocarcinoma (5.8%, n=43), and hyperplastic/inflammatory polyp (2.1%, n=16) (Supplementary Table 1).
Ten studies were prospective, four were retrospective cohort studies, and two were randomized trials. Among the 14 cohort studies, 9 were of high quality [16-18,40,43-47] and 5 were of medium quality [20,39,41,42,48]. Both randomized trials were of good quality [49,50]. The quality assessment results are shown in Supplementary Table 2.
The follow-up period ranged from 3 to 13 months, and a total of 584 patients were followed up in 10 studies. The pooled rate of residual/recurrent polyps on the first follow-up was 7.3% (95% confidence interval [CI], 4.3–12.0; I2=52%) with moderate heterogeneity (Fig. 2A).
The pooled rate of en bloc resection was 57.7% (95% CI, 42.4–71.6; I2=95%) with considerable heterogeneity (Fig. 2B). R0 resection was reported in eight studies, and the pooled rate was 58.9% (95% CI, 42.4–73.6; I2=92%) (Fig. 2C). Eleven studies reported a rate of incomplete macroscopic resection of 1.5% (95% CI, 0.8–2.7; I2=0%) with no heterogeneity (Fig. 2D).
The pooled rate of any adverse event after UEMR was 7.0% (95% CI, 4.7–10.3; I2=66%) with substantial heterogeneity (Fig. 3A). A total of 109 adverse events were reported. The most common adverse event was intraprocedural bleeding (n =79), followed by delayed bleeding (n =25), perforation (n=1), and PPS (n=1). In one study, the authors also reported three cases (2.7%) of muscle-layer injury without perforation that were successfully treated with endoscopic clipping [42]. The pooled rates of intraprocedural and delayed bleeding were 5.4% (95% CI, 3.3–8.8; I2=64%) and 2.9% (95% CI, 2.0–4.1; I2=0%), respectively (Fig. 3B, C). Only one case of perforation and one case of PPS were reported in the 16 studies, and the pooled rate was 0.8% (95% CI, 0.4–1.6; I2=0%) with no heterogeneity (Fig 3D). These results are summarized in Table 3.
Ten studies with a total of 489 nonpedunculated polyps ≥20 mm reported the outcomes of UEMR. The mean size was 28.5 ±7.1 mm (range 22.5–38.0 mm). The data on assessed UEMR outcomes for nonpedunculated polyps ≥20 mm are shown in Supplementary Table 3. A total of 325 patients were followed up for a range of 3–8 months in four studies. The recurrence/residual polyp rate was 5.9% (95% CI, 3.6–9.4; I2=0). The pooled rate of en bloc, incomplete, and R0 resection was 41.3% (95% CI, 27.8–56.4; I2=83%), 1.8% (95% CI, 0.8–4.1; I2=0%), and 48.8% (95% CI, 37.1–60.6; I2=21.6%), respectively. The pooled rate of any adverse events, intraprocedural bleeding, and delayed bleeding was 12.5% (95% CI, 6.5–22.5; I2=55%), 10.5% (95% CI, 5.2–20.1; I2=56.5%), and 2.5% (95% CI, 1.3–4.6; I2=0%), respectively. There was no case of perforation and only one case of PPS with a rate of 1.7% (95% CI, 0.7–3.9; I2= 0%) in this subgroup (Table 3).
Meta-regression was performed for recurrence rate, adverse events, and intraprocedural bleeding. The included variables were percentage of proximally located polyps, mean polyp size, mean age, and study type (prospective and retrospective). The only significant and positive predictor was proximal polyp location, with a regression coefficient of 0.03 (95% CI, 0.005–0.064; p=0.02) for all adverse events and a regression coefficient of 0.04 (95% CI, 0.006–0.07; p=0.01) for intraprocedural bleeding. Proximal lesions also accounted for heterogeneity in our study outcomes, with R2 (accounted heterogeneity) of 34.5%, 34.3%, and 61.6% for all adverse events, intraprocedural bleeding, and recurrence rate, respectively. Mean patient age, polyp size, and study type did not have any significant predictive influence on these outcomes. The results of meta-regression are summarized in Supplementary Table 4. A scatterplot showing the relationship between proximal lesions and adverse events is also shown in Supplementary Fig. 1.
We excluded one study at a time and analyzed its effect on the main summary estimate to assess the dominant effect of any outcome on the meta-analysis. No single study significantly affected the outcome on sensitivity analysis.
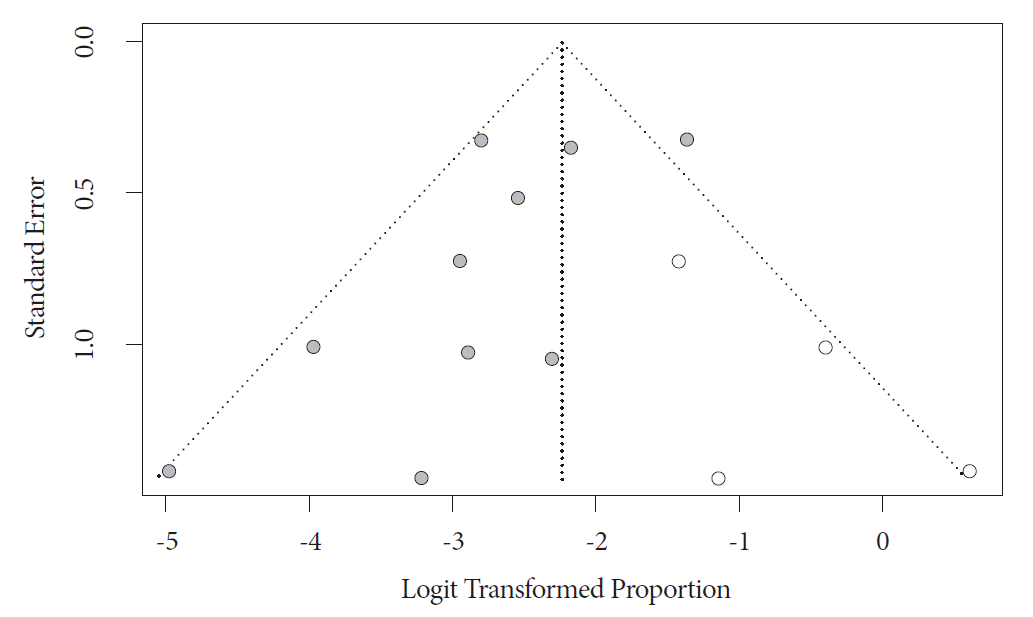
Evidence of publication bias was found on visual inspection of the funnel plot and on quantitative measurement using the Egger regression test (Egger’s two-tailed p-value =0.02). On further trim-and-fill analysis, four studies were added, which adjusted our primary outcome of recurrence rate to 9.7% (95% CI, 5.9–15.6). On the basis of overlapping CI with our primary outcome, the impact of publication bias was considered modest. The funnel plot with added studies is shown in Fig. 4.
Our study demonstrates that UEMR for nonpedunculated polyps ≥10 mm in size has high efficacy with low rates of recurrence (7.3%) and incomplete resection (1.5%), and high rates of en bloc resection (57.7%) and R0 resection (58.9%). The overall adverse event rate of 7.0% after UEMR is encouraging, with low rates of intraprocedural bleeding (5.4%) and delayed bleeding (2.9%), and a <1% incidence of procedure-related perforation and PPS. The rate of incomplete resection and recurrence for large polyps ≥20 mm was 1.8% and 5.9%, respectively. Our study is the largest and first meta-analysis to report the outcomes of UEMR for nonpedunculated polyps ≥10 mm in size.
The rate of en bloc resection after CEMR was reported to be 38%–46.7% in previous large studies [54,55]. The rate of en bloc resection was 58% in our study, which is better than the reported rate with CEMR. In addition, the high R0 resection rate (59%) coupled with very low rates of incomplete resection (1.5%) after UEMR is also highly relevant, especially for large polyps. A randomized trial comparing UEMR and CEMR found significantly higher rates of en bloc resection (89% vs. 75%, p=0.001) and R0 resection (69% vs. 50%, p=0.01) with UEMR for nonpedunculated polyps 10–19 mm [49]. Schenck et al. also reported a higher rate of complete resection with UEMR than with CEMR [46]. The colonic wall assumes its collapsed state underwater, which enables more efficient and superior snare capture of large lesions during UEMR. The superior snare capture and the absence of submucosal injection likely explain the high rates of complete resection, en bloc resection, and R0 resection.
The other major finding of our study was the very low residual/recurrent polyp rate of 7.3% after UEMR. In a large meta-analysis of nonpedunculated polyps, the authors reported an overall recurrence rate of 15% after CEMR after a follow-up of 3–6 months [56]. The authors also found that piecemeal resection was associated with a higher risk of local recurrence than en bloc resection [56]. Another multicenter prospective study reported a recurrence rate of 16% after CEMR [14]. On multivariate analysis, large polyp size ≥40 mm and intraprocedural bleeding were associated with a high risk of recurrence. However, another meta-analysis of only colorectal polyps ≥20 mm reported a recurrence rate of 13.8% after CEMR [55]. Interestingly, we found that the recurrence rate was even lower (5.9%) for large nonpedunculated polyps ≥20 mm. This finding is highly relevant in real-world settings, as follow-up is highly variable and dependent on patient compliance. In our study itself, the compliance was only 70% after including only studies that reported follow-up data. UEMR with a relative risk reduction of 6.5% (13.8% CEMR – 7.3% UEMR), by modest measures and taking into account a 70% compliance rate, will potentially prevent around two recurrences for every 100 patients that would otherwise be missed and can have malignant transformation. These estimates will have a large impact on healthcare utilization when applied to large populations. We speculate that the high complete resection rate of UEMR is likely to translate into a low rate of recurrence.
The rate of intraprocedural bleeding and delayed bleeding associated with CEMR has been reported to be in the range of 6.5%–11.3% and 2.6%–6.0%, respectively [57,58]. The adverse event rates of UEMR are similar to those of CEMR, but the risk of perforation is extremely low with UEMR. In our study, UEMR was associated with only one case each of perforation and PPS. There was only one perforation among >1,000 cases of UEMR, whereas the risk of perforation after CEMR has been reported to be 1%–2% in previous studies [57]. This is likely due to foregoing the submucosal injection step in UEMR, which is typically performed in CEMR, thus minimizing injury to the muscle layer. Our results suggest that UEMR is a highly safe procedure for the removal of large nonpedunculated polyps. On meta-regression, proximal lesions had a positive predictive influence on adverse events and intraprocedural bleeding, which is likely due to the technical challenges during the procedure for such lesions.
UEMR might have advantages over CEMR, including a lower resection time. In a randomized trial, the procedure duration was significantly lower with UEMR than with CEMR for lesions 10–19 mm (2.9 vs. 5.6 min, p<0.0001) and ≥20 mm (7.3 vs. 9.5 min, p=0.015) in size [50]. Procedure duration is important for clinicians with busy practice. The shorter procedure duration could be explained by the absence of submucosal injection and the low rate of intraprocedural bleeding requiring endoscopic hemostasis. The presence of a thin wall at the appendiceal orifice and ileocecal valve makes CEMR technically challenging. However, even for these locations, high complete resection rates and low adverse event rates have been reported with UEMR [52,59]. In addition, UEMR could provide additional value in the resection of recurrent lesions. Kim et al. reported that UEMR is superior in terms of a higher rate of en bloc resection and fewer adenoma recurrences than CEMR [51]. Although still early, the data and future prospects of UEMR for difficult and recurrent lesions certainly seem promising.
As mentioned before, ESD is considered the treatment of choice for lesions > 20 mm with a Paris IIc or IIa + IIc morphology or for any lesions >3 cm [15]. It is, however, limited by its high complication rate of 2%–14% [60]. ESD has a very slow uptake in North America primarily owing to its slow learning curve and the need for extensive training even by experienced endoscopists [2,61,62]. ESD is also technically challenging and time consuming. In contrast, endoscopists experienced in CEMR can easily learn UEMR, and it can have widespread application in the community.
Our review has several strengths, including the systematic literature search with well-defined inclusion criteria, careful exclusion of redundant studies, inclusion of good-quality studies with detailed extraction of data, and rigorous evaluation of study quality. The studies also included flat lesions. Some of the included studies exclusively analyzed patients with large polyps. The included studies were also representative of the general population and community practice. Meanwhile, there are limitations to this study, most of which are inherent to any meta-analysis. Our analysis included retrospective studies, thus contributing to selection bias. We were unable to directly compare UEMR with another endoscopic method owing to paucity of data. Long-term data on polyp recurrence are also lacking at this time. We were unable to further differentiate polyps into the Is, II, and III groups. Nevertheless, our study is the best available estimate and first meta-analysis in the literature reporting the clinical outcomes of UEMR for nonpedunculated polyps ≥10 mm.
In conclusion, our meta-analysis demonstrates that UEMR for nonpedunculated colorectal polyps ≥10 mm in size is safe and effective with a low recurrence rate.
Supplementary Material
Supplementary Fig. 1.
Scatterplot showing the relationship of proximal lesions and adverse events on meta-regression.
Supplementary Table 1.
Data on Histopathology of Polyps Removed Using Underwater Endoscopic Mucosal Resection
Supplementary Table 2A.
Study Quality Assessment Based on Newcastle-Ottawa Scale
Supplementary Table 2B.
Showing Study Quality Assessment Based on Jadad Score for Randomized Controlled Trials
Supplementary Table 3.
Data on Assessed Outcomes for Nonpedunculated Polyps ≥20 mm
Notes
Author Contributions
Conceptualization: Rajat Garg, Amandeep Singh, Prabhleen ChahalC
Data curation: RG, Manik Aggarwal, Jaideep BhallaC
Formal analysis: RGC
Investigation: RGC
Methodology: RG, Babu P. MohanC
Supervision: PCC
Writing-original draft: RG, ASC
Writing-review & editing: RG, AS, MA, JB, BPM, Carol Burke, Tarun Rustagi, PC
ACKNOWLEDGMENTS
The authors thank medical librarian Marian T. Simonson, MSLS, AHIP, for assisting in the literature search.
REFERENCES
1. National Cancer Institute. Cancer stat facts: colorectal cancer [Internet]. Rockville (MD): SEER;c2021. [cited 2021 Mar 31]. Available from: https://seer.cancer.gov/statfacts/html/colorect.html.
2. Gaglia A, Sarkar S. Evaluation and long-term outcomes of the different modalities used in colonic endoscopic mucosal resection. Ann Gastroenterol. 2017; 30:145–151.

3. Holt BA, Bourke MJ. Wide field endoscopic resection for advanced colonic mucosal neoplasia: current status and future directions. Clin Gastroenterol Hepatol. 2012; 10:969–979.

4. Bourke MJ. Endoscopic resection for mucosal neoplasia: pushing the boundaries, confronting the reality. J Gastroenterol Hepatol. 2011; 26:1582–1584.

5. Ferlitsch M, Moss A, Hassan C, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017; 49:270–297.

6. Kaltenbach T, Anderson JC, Burke CA, et al. Endoscopic removal of colorectal lesions-recommendations by the US multi-society task force on colorectal cancer. Gastroenterology. 2020; 158:1095–1129.

7. Binmoeller KF, Bohnacker S, Seifert H, Thonke F, Valdeyar H, Soehendra N. Endoscopic snare excision of “giant” colorectal polyps. Gastrointest Endosc. 1996; 43:183–188.

8. Iishi H, Tatsuta M, Iseki K, et al. Endoscopic piecemeal resection with submucosal saline injection of large sessile colorectal polyps. Gastrointest Endosc. 2000; 51:697–700.

9. Swan MP, Bourke MJ, Moss A, Williams SJ, Hopper A, Metz A. The target sign: an endoscopic marker for the resection of the muscularis propria and potential perforation during colonic endoscopic mucosal resection. Gastrointest Endosc. 2011; 73:79–85.

10. Knabe M, Pohl J, Gerges C, Ell C, Neuhaus H, Schumacher B. Standardized long-term follow-up after endoscopic resection of large, nonpedunculated colorectal lesions: a prospective two-center study. Am J Gastroenterol. 2014; 109:183–189.

11. Swan MP, Bourke MJ, Alexander S, Moss A, Williams SJ. Large refractory colonic polyps: is it time to change our practice? a prospective study of the clinical and economic impact of a tertiary referral colonic mucosal resection and polypectomy service (with videos). Gastrointest Endosc. 2009; 70:1128–1136.

12. Jayanna M, Burgess NG, Singh R, et al. Cost analysis of endoscopic mucosal resection vs surgery for large laterally spreading colorectal lesions. Clin Gastroenterol Hepatol. 2016; 14:271–278.e1-e2.
13. Fukami N, Lee JH. Endoscopic treatment of large sessile and flat colorectal lesions. Curr Opin Gastroenterol. 2006; 22:54–59.

14. Moss A, Williams SJ, Hourigan LF, et al. Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut. 2015; 64:57–65.

15. Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015; 27:417–434.

16. Binmoeller KF, Weilert F, Shah J, Bhat Y, Kane S. “Underwater” EMR without submucosal injection for large sessile colorectal polyps (with video). Gastrointest Endosc. 2012; 75:1086–1091.

17. Barclay RL, Percy DB. Underwater endoscopic mucosal resection without submucosal injection (UEMR) for large colorectal polyps: a community-based series. Am J Surg. 2020; 220:693–696.

18. Binmoeller KF, Hamerski CM, Shah JN, Bhat YM, Kane SD. Garcia-Kennedy R Attempted underwater en bloc resection for large (2-4 cm) colorectal laterally spreading tumors (with video). Gastrointest Endosc. 2015; 81:713–718.
19. Nett A, Binmoeller K. Underwater Endoscopic Mucosal Resection. Gastrointest Endosc Clin N Am. 2019; 29:659–673.

20. Wang AY, Flynn MM, Patrie JT, et al. Underwater endoscopic mucosal resection of colorectal neoplasia is easily learned, efficacious, and safe. Surg Endosc. 2014; 28:1348–1354.

21. Spadaccini M, Fuccio L, Lamonaca L, et al. Underwater EMR for colorectal lesions: a systematic review with meta-analysis (with video). Gastrointest Endosc. 2019; 89:1109–1116.e4.

22. Li DF, Lai MG, Yang MF, et al. The efficacy and safety of underwater endoscopic mucosal resection for ≥10-mm colorectal polyps: systematic review and meta-analysis. Endoscopy. 2020; Aug. 6. [Epub]. https://doi.org/10.1055/a-1234-8918.

23. Li P, Ma B, Gong S, Zhang X, Li W. Underwater endoscopic mucosal resection for colorectal lesions: a meta-analysis. Surg Endosc. 2020; Jun. 23. [Epub]. https://doi.org/10.1007/s00464-020-07745-8.

24. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009; 151:264–269.W64.

25. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000; 283:2008–2012.
26. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010; 25:603–605.

27. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996; 17:1–12.

29. Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical research. Hoboken (NJ): John Wiley & Sons;2000. p. 205–228.
30. Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009; 172:137–159.

31. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011; 342:d549.

32. Mohan BP, Adler DG. Heterogeneity in systematic review and meta-analysis: how to read between the numbers. Gastrointest Endosc. 2019; 89:902–903.

33. Kanwal F, White D. “Systematic Reviews and Meta-analyses” in Clinical Gastroenterology and Hepatology. Clin Gastroenterol Hepatol. 2012; 10:1184–1186.

34. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560.

35. Guyatt GH, , Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence--inconsistency. J Clin Epidemiol. 2011; 64:1294–1302.

36. Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991; 337:867–872.

37. Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000; 56:455–463.

38. Rothstein HR, Sutton AJ, Borenstein M. Publication bias in meta-analysis: Prevention, assessment and adjustments. Hoboken (NJ): John Wiley & Sons;2006.
39. Amato A, Radaelli F, Spinzi G. Underwater endoscopic mucosal resection: The third way for en bloc resection of colonic lesions? United European Gastroenterol J. 2016; 4:595–598.

40. Cadoni S, Liggi M, Gallittu P, et al. Underwater endoscopic colorectal polyp resection: feasibility in everyday clinical practice. United European Gastroenterol J. 2018; 6:454–462.

41. Chaves DM, Brito HP, Chaves LT, Rodrigues RA, Sugai BM. Underwater endoscopic mucosal resection of serrated adenomas. Clinics (Sao Paulo). 2018; 73:e339.

42. Chien H-C, Uedo N, Hsieh P-H. Comparison of underwater and conventional endoscopic mucosal resection for removing sessile colorectal polyps: a propensity-score matched cohort study. Endosc Int Open. 2019; 07:E1528–E1536.

43. Curcio G, Granata A, Ligresti D, et al. Underwater colorectal EMR: remodeling endoscopic mucosal resection. Gastrointest Endosc. 2015; 81:1238–1242.

44. Rodriguez Sanchez J, Uchima Koecklin H, Gonzalez Lopez L, et al. Short and long-term outcomes of underwater EMR compared to the traditional procedure in the real clinical practice. Rev Esp Enferm Dig. 2019; 111:543–549.

45. Sandhu DS, Lee YJ, Gerke H. Underwater endoscopic mucosal resection: an alternative treatment for large colorectal polyp removal. Minerva Gastroenterol Dietol. 2018; 64:106–110.

46. Schenck RJ, Jahann DA, Patrie JT, et al. Underwater endoscopic mucosal resection is associated with fewer recurrences and earlier curative resections compared to conventional endoscopic mucosal resection for large colorectal polyps. Surg Endosc. 2017; 31:4174–4183.

47. Siau K, Ishaq S, Cadoni S, Kuwai T, Yusuf A, Suzuki N. Feasibility and outcomes of underwater endoscopic mucosal resection for ≥10 mm colorectal polyps. Surg Endosc. 2018; 32:2656–2663.
48. Uedo N, Nemeth A, Johansson GW, Toth E, Thorlacius H. Underwater endoscopic mucosal resection of large colorectal lesions. Endoscopy. 2015; 47:172–174.

49. Yamashina T, Uedo N, Akasaka T, et al. Comparison of underwater vs conventional endoscopic mucosal resection of intermediate-size colorectal polyps. Gastroenterology. 2019; 157:451–461.e2.

50. Yen AW, Leung JW, Wilson MD, Leung FW. Underwater versus conventional endoscopic resection of nondiminutive nonpedunculated colorectal lesions: a prospective randomized controlled trial (with video). Gastrointest Endosc. 2020; 91:643–654.e2.

51. Kim HG, Thosani N, Banerjee S, Chen A, Friedland S. Underwater
endoscopic mucosal resection for recurrences after previous piecemeal resection of colorectal polyps (with video). Gastrointest Endosc. 2014; 80:1094–1102.
52. Binmoeller KF, Hamerski CM, Shah JN, Bhat YM, Kane SD. Underwater EMR of adenomas of the appendiceal orifice (with video). Gastrointest Endosc. 2016; 83:638–642.
53. Kawamura T, Sakai H, Ogawa T, et al. Feasibility of underwater endoscopic mucosal resection for colorectal lesions: a single center study in Japan. Gastroenterology Res. 2018; 11:274–279.

54. Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015; 81:583–595.

55. Hassan C, Repici A, Sharma P, et al. Efficacy and safety of endoscopic resection of large colorectal polyps: a systematic review and meta-analysis. Gut. 2016; 65:806–820.

56. Belderbos TDG, Leenders M, Moons LMG, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy. 2014; 46:388–402.

57. Kandel P, Wallace MB. Colorectal endoscopic mucosal resection (EMR). Best Pract Res Clin Gastroenterol. 2017; 31:455–471.

58. Klein A, Tate DJ, Jayasekeran V, et al. Thermal ablation of mucosal defect margins reduces adenoma recurrence after colonic endoscopic mucosal resection. Gastroenterology. 2019; 156:604–613.e3.

59. Levy I, Hamerski CM, Nett AS, Calitis J, Binmoeller KF. Su1618 underwater endoscopic mucosal resection (UEMR) of laterally spreading tumors involving the ileocecal valve. Gastrointestinal Endoscopy. 2017; 85:AB366.

60. Nakajima T, Saito Y, Tanaka S, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc. 2013; 27:3262–3270.

Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram showing the search strategy for meta-analysis. CI, confidence interval.
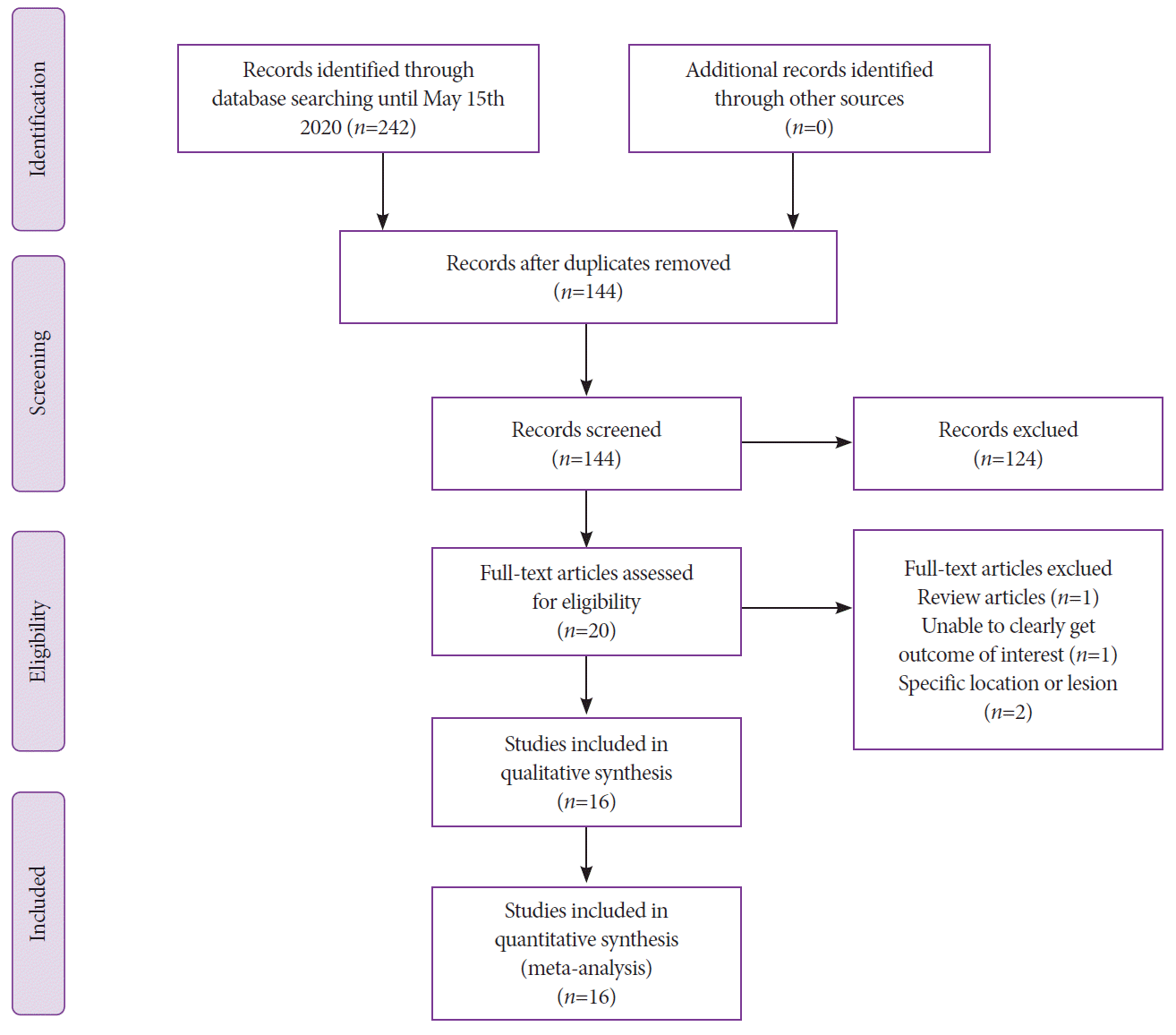
Fig. 2.
Nonpedunculated polyps ≥10 mm after underwater endoscopic mucosal resection. (A) Forest plot showing the pooled rates of recurrence. (B) En bloc resection. (C) R0 resection. (D) Incomplete resection. CI, confidence interval.
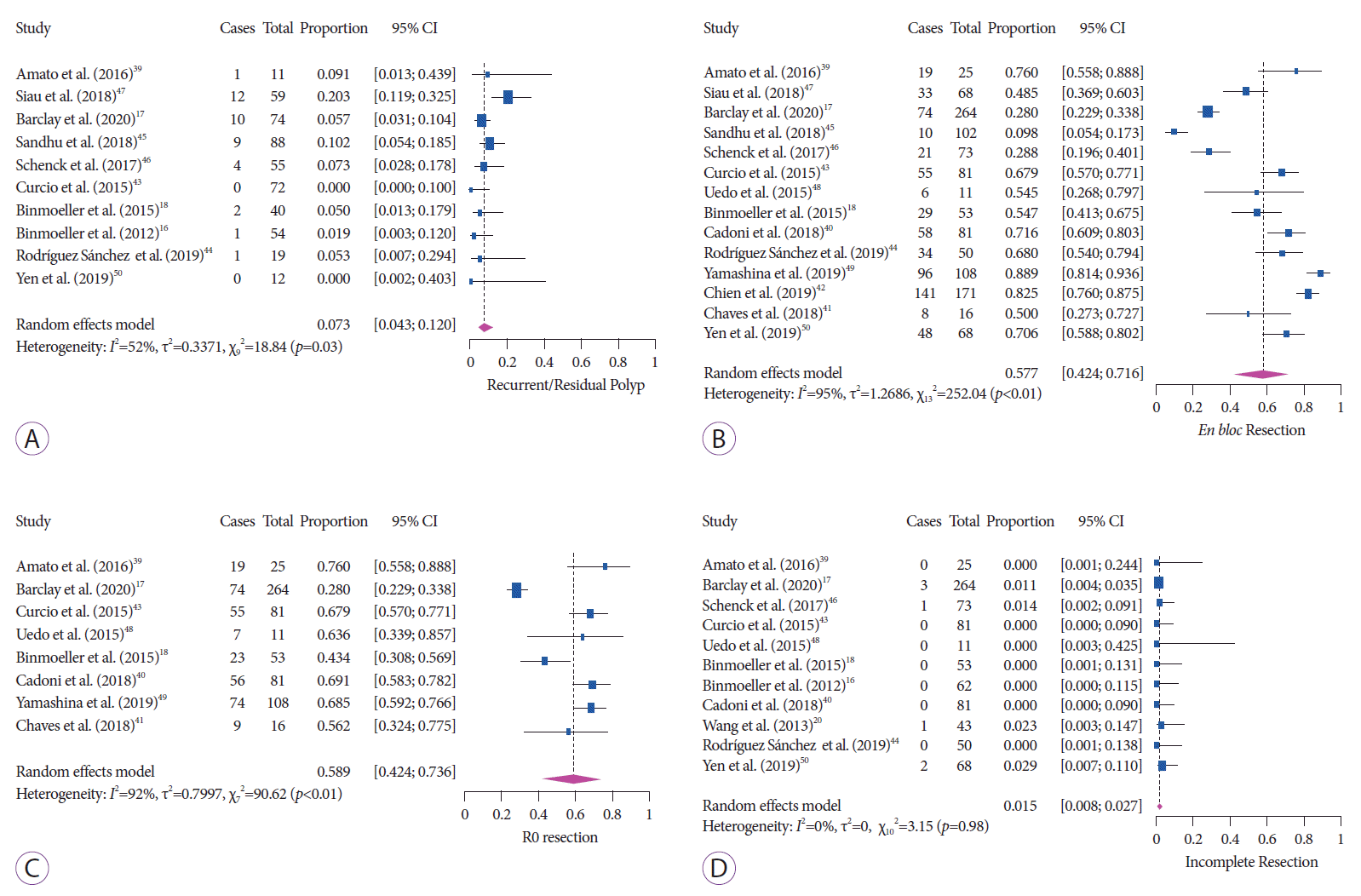
Fig. 3.
Underwater endoscopic mucosal resection for nonpedunculated polyps ≥10 mm. (A) Forest plot showing the rates of adverse events. (B) Intraprocedural bleeding. (C) Delayed bleeding. (D) Perforation.
Table 1.
Study and Population Characteristics
| Study | Study type | Age,a mean± SD or median (yr) | Number of patients | Number of polyps | Female (n) | Mean polyp sizea (mm) | Proximal lesions (n) | Mean resection timea (min) |
|---|---|---|---|---|---|---|---|---|
| Amato et al. (2016) [39] | Prospective | 62.2 | 25 | 25 | 22.8 | 18 | 24.6 | |
| Siau et al. (2018) [47] | Prospective | 69.5±11 | 85 | 68 | 36 | 20±20.8 | 25 | |
| Barclay et al. (2020) [17] | Prospective | 67±9 | 242 | 264 | 99 | 38±18 | 193 | 13.7±10.6 |
| Sandhu et al. (2018) [45] | Retrospective | 64.7±9.7 | 93 | 102 | NR | 20.4±9.4 | 80 | NR |
| Schenck et al. (2017) [46] | Retrospective | 64.1±12.3 | 46 | 73 | 19 | 25.4 | 49 | NR |
| Curcio et al. (2015) [43] | Prospective | 66.9 | 72 | 81 | 26 | 18.6 | 49 | 11.8 |
| Uedo et al. (2015) [48] | Prospective | 75±8.7 | 11 | 11 | 3 | 18.6±3.2 | 10 | NR |
| Binmoeller et al. (2015) [18] | Prospective | 68 (50–88) | 50 | 53 | 25 | 30 (20–40) | 38 | 38 (17–87) |
| Binmoeller et al. (2012) [16] | Prospective | 63.8 (46.6–81) | 60 | 62 | 32 | 30 (15–45) | 38 | 21.4 |
| Cadoni et al. (2018) [40] | Retrospective | 64.7 (9) | 146 | 81 | 45 | 10 (9.25–15) | 67 | 2 (0.8–5) |
| Wang et al. (2013) [20] | Prospective | 64.9 (51–83) | 21 | 43 | 4 | 20 (8–70) | 21 | 12.7 (2–48) |
| Rodríguez Sánchez et al. (2019) [44] | Prospective | 66.25±10.53 | NR | 50 | NR | 20.78 (15–50) | 38 | NR |
| Yamashina et al. (2019) [49] | RCT | 70 (43–86) | 108 | 108 | 40.7 | 14 (7–25) | 66 | NR |
| Chien et al. (2019) [42] | Retrospective | 63.4 (9.9) | 158 | 171 | 63 | 15.8 (6) | 94 | 9.7±7.7 |
| Chaves et al. (2018) [41] | Prospective | 54.5 (48–72) | 14 | 16 | 10 | 20 (10–35) | 15 | NR |
| Yen et al. (2019) [50] | RCT | 64.4±8.3 | 128 | 68 | 2 | 9.9±6.4 | 51 | 3.8±0.34 |
Table 2.
Data on Assessed Outcomes of Underwater Endoscopic Mucosal Resection for Nonpedunculated Polyps ≥10 mm
| Study | Number of polyps (n) | En bloc resection (n) | Incomplete resection (n) | R0 resection (n) | Total complications (n) | Intraprocedural bleeding (n) | Delayed bleeding (n) | Perforation (n) | Postpolypectomy syndrome (n) | Recurrence per patient on follow up |
|---|---|---|---|---|---|---|---|---|---|---|
| Amato et al. (2016) [39] | 25 | 19 | 0 | 19 | 2 | 2 | 0 | 0 | 0 | 1/11 |
| Siau et al. (2018) [47] | 68a | 33 | NR | NR | 2 | 2 | 0 | 0 | 0 | 12/59 |
| Barclay et al. (2020) [17] | 264 | 74 | 3 | 74 | 44 | 39 | 4 | 0 | 1 | 10/174 |
| Sandhu et al. (2018) [45] | 102 | 10 | NR | NR | 15 | 9 | 7 | 0 | 0 | 9/88 |
| Schenck et al. (2017) [46] | 73 | 21 | 1 | NR | 3 | 0 | 3 | 0 | 0 | 4/55 |
| Curcio et al. (2015) [43] | 81 | 55 | 0 | 55 | 0 | 0 | 0 | 0 | 0 | 0/72 |
| Uedo et al. (2015) [48] | 11 | 6 | 0 | 7 | 2 | 2 | 0 | 0 | 0 | NR |
| Binmoeller et al. (2015) [18] | 53 | 29 | 0 | 23 | 1 | 0 | 1 | 0 | 0 | 2/40 |
| Binmoeller et al. (2012) [16] | 62 | NR | 0 | NR | 3 | 0 | 3 | 0 | 0 | 1/54 |
| Cadoni et al. (2018) [40] | 81 | 58 | 0 | 56 | 11b | 10 | 1 | 0 | 0 | NR |
| Wang et al. (2013) [20] | 43 | NR | 1 | NR | 1 | 0 | 1 | 0 | 0 | NR |
| Rodríguez Sánchez et al. (2019) [44] | 50 | 34 | 0 | NR | 1 | 1 | 0 | 0 | 0 | 1/19 |
| Yamashina et al. (2019) [49] | 108 | 96 | NR | 74 | 3 | 0 | 3 | 0 | 0 | NR |
| Chien et al. (2019) [42] | 171 | 141 | NR | NR | 15b | 9 | 2 | 1 | 0 | NR |
| Chaves et al. (2018) [41] | 16 | 8 | NR | 9 | 0 | 0 | 0 | 0 | 0 | NR |
| Yen et al. (2019) [50] | 68c | 48 | 2 | - | 5 | 5 | 0 | 0 | 0 | 0/12 |
Table 3.
Meta-Analysis Results of UEMR for Nonpedunculated Polyps ≥ 10 and ≥ 20 mm
| Nonpedunculated polyps ≥10 mma | Nonpedunculated polyps ≥20 mma | |
|---|---|---|
| Recurrence/residual polyp | 7.3% (4.3–12; 52%), 10 studies | 5.9% (3.6–9.4; 0%), 4 studies |
| En bloc resection | 57.7% (42.4–71.6; 95%), 14 studies | 41.3% (27.8–56.4; 83%), 10 studies |
| Incomplete resection | 1.5% (0.8–2.7; 0%), 11 studies | 1.8% (0.8–4.1; 0%), 5 studies |
| R0 resection | 58.9% (42.4–73.6; 92%), 8 studies | 48.8% (37.1–60.6; 21.6%), 5 studies |
| Total adverse events | 7.0% (4.7–10.3; 66%), 16 studies | 12.5% (6.5–22.5; 55.2%), 6 studies |
| Intraprocedural bleeding | 5.4% (3.3–8.8; 64%), 16 studies | 10.5% (5.2–20.1; 56.5%), 7 studies |
| Delayed bleeding | 2.9% (2.0–4.1; 0%), 16 studies | 2.5% (1.3–4.6; 0%), 8 studies |
| Perforation | 0.8% (0.4–1.6; 0), 16 studies | NA |
| Postpolypectomy syndrome | 0.8% (0.4–1.5; 0%), 16 studies | 1.7% (0.7–3.9; 0%), 10 studies |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download