Abstract
Background/Aims
To date, no reports have compared the diagnostic efficacy of narrow-band imaging (NBI) and i-scan for the histologic prediction of intermediate-to-large colorectal polyps. We aimed to compare the diagnostic accuracy of NBI and i-scan in predicting histology, and their inter-/intra-observer agreement.
Methods
We performed a prospective, randomized study that included 66 patients (NBI, n=33 vs. i-scan, n=33) with colorectal polyps (size >10 mm but <50 mm) who underwent colonoscopic resection. During the procedure, three endoscopists documented their prediction using the Japan NBI Expert Team (JNET) classification. Two months after study completion, the endoscopists reviewed still images and video clips for analysis.
Results
The overall diagnostic accuracies in the NBI and i-scan groups were 73.7% (73/99) and 75.8% (75/99), respectively, and there was no statistical significance between the two groups (p=0.744). The JNET classification as applied to NBI and i-scan showed substantial inter-observer agreement (NBI κ-value 0.612, p=0.001 vs. i-scan κ-value 0.662, p=0.002). Additionally, the κ-values of intra-observer agreement were in the range of 0.385–0.660 with NBI and 0.364–0.741 with i-scan.
Colonoscopy is considered an optimal modality for the detection and treatment of colorectal neoplasms [1]. In most cases, colorectal cancer (CRC) arises from adenomatous polyps according to the adenoma–carcinoma sequence [2]. Therefore, the removal of colorectal adenomatous polyps through colonoscopic polypectomy can reduce the risk of progression to CRC [3]. For this reason, an accurate optical diagnosis during colonoscopy is of utmost importance because it can avoid unnecessary treatment-related procedures and reduce high medical costs. The current-generation colonoscopes and processors provide high-resolution images of the colorectal mucosa, enabling the detailed inspection of lesions. However, the accuracy of whitelight endoscopy (WLE) is not adequate for the prediction of histology, as it cannot satisfactorily distinguish subtle mucosal abnormalities. In this regard, chromoendoscopic techniques, such as dye-spray chromoendoscopy and image-enhanced endoscopy (IEE), have been introduced to distinguish among various mucosal abnormalities [4,5]. The combination of magnifying colonoscopy and dye-spray chromoendoscopy has been reported to be reliable for distinguishing colorectal neoplasms [6]. However, dye-spray chromoendoscopy requires the additional procedure of dye spraying, which increases the cost and time required for the procedure. In contrast, IEE techniques can assist with the real-time detection and assessment of colorectal neoplasms with simple activation during colonoscopy [7].
Narrow-band imaging (NBI; Olympus, Tokyo, Japan) is an IEE technique that has been widely used in clinical practice. NBI is a method of image enhancement that modifies white light via narrowed bandwidth filters to allow the visualization of the surface pattern and capillary vessels. This enables endoscopists to identify neoplastic lesions [8]. To date, many classifications based on the NBI system have been suggested. Recently, the Japan NBI Expert Team (JNET) introduced a new classification system [9]. This system categorizes colorectal neoplasms into four types (types 1, 2A, 2B, and 3) based on surface patterns and capillary vessels [9]. Another IEE technique, i-scan (Pentax, Tokyo, Japan), was introduced as a diagnostic modality for the differentiation of colorectal neoplasms. This method uses post-processing computer algorithms to enhance the visibility of surface structures and vascular patterns. The i-scan technology is a digital contrast method with three adjustable modes of image enhancement (surface enhancement [SE], contrast enhancement [CE], and tone enhancement [TE]) [10,11].
One previous report compared the diagnostic efficacy of NBI and i-scan in terms of their ability to predict the histology of diminutive colonic polyps [12]. However, no studies have compared the diagnostic efficacy of NBI and i-scan for the histologic prediction of intermediate-to-large colorectal polyps. The optical prediction of histology is crucial because intermediate-to-large colorectal polyps tend to exhibit advanced histology [13]. Therefore, the aims of the current prospective, randomized pilot study were to compare the diagnostic accuracy of NBI and i-scan in terms of their ability to predict histology, and to compare their inter-/intra-observer agreement based on the JNET classification in intermediate-to-large colorectal polyps.
We performed a prospective, randomized pilot study to compare NBI and i-scan for the prediction of the histology of colorectal polyps during colonoscopic resection. A total of 66 patients (one lesion per patient) who underwent colonoscopic resection for intermediate-to-large colorectal polyps between December 2019 and June 2020 were considered eligible. The inclusion criteria were as follows: (1) age >18 years, (2) colonoscopic resection for the treatment of colorectal polyps, (3) polyp size >10 mm but <50 mm, and (4) no underlying disease that is associated with a higher likelihood of bleeding. The exclusion criteria were as follows: (1) previous diagnosis of a colorectal malignancy, familial adenomatous polyposis, or inflammatory bowel disease; (2) history of diverticulosis and radiation treatment; (3) pregnant status in women; and (4) refusal to participate in this study. All procedures were performed by three experienced (>5 years of therapeutic endoscopy experience) endoscopists (JSL, SWJ, and YHK) who were familiar with the IEE modalities of NBI and i-scan. The study was reviewed and approved by the institutional review board of Kyungpook National University Hospital (KNUCH 2019-06-017).
We adapted the JNET classification for histologic prediction. JNET type 1 is characterized by changes in normal vessels and mucosal patterns, as well as the presence of dark and white spots. Type 1 indicates a hyperplastic polyp (HP) or a sessile serrated adenoma/polyp (SSA/P). Type 2A is characterized by vessels of a regular caliber and a normal distribution pattern. Type 2A indicates low-grade dysplasia (LGD) and includes tubular adenoma and tubulovillous adenoma. Type 2B is characterized by vessels of a variable caliber with an irregular distribution pattern and an obscure mucosal pattern. Type 2B indicates high-grade dysplasia (HGD), intramucosal cancer (IMC), and superficial submucosal invasive cancer (sSMIC). Type 3 is defined as a vessel pattern that is typified by areas of interrupted thick vessels and a mucosal pattern involving amorphous areas. Type 3 indicates deep submucosal invasive cancer (dSMIC) [9]. Representative NBI images obtained from the enrolled patients, presented according to the JNET classification, are shown in Fig. 1.
The i-scan method combines high-resolution colonoscopy with three adjustable modes of image enhancement: SE, CE, and TE. The TE mode is similar to NBI and may be suitable for the characterization and prediction of the histology of colorectal polyps [10]. Therefore, we adapted the TE(c) mode, which is the optimal i-scan setting for colonoscopy, as recommended by the manufacturer. Thus far, no validated classifications of colorectal polyps with the use of i-scan have been reported. We applied the JNET classification to predict histology. Representative i-scan images from eligible patients to which the JNET classification was applied are shown in Fig. 2.
Before study initiation, the three endoscopists participated in an interactive training session that included 20 representative images. The images comprised five images of each of the four JNET classifications acquired with NBI and i-scan. The patients were randomly assigned to the NBI group (n=33) or the i-scan group (n=33). The endoscopists evaluated the detected lesions with WLE and IEE (NBI or i-scan) before performing colonoscopic resection. The still images and video clips from WLE and IEE were obtained for analysis. All images were obtained using commercially available high-definition colonoscopes (CF-H180AL/I [Olympus Optical Co., Tokyo, Japan] or EC38-i10c [Pentax Medical, Tokyo, Japan]) equipped with video processors (EVIS EXERA II [Olympus Optical Co.] or IMAGINA EPK-i5500c [Pentax Medical]). After the assessment, the endoscopists recorded their prediction and the JNET classification of colorectal polyps in the case report form. Subsequently, the samples (endoscopically or surgically resected) were reviewed by experienced gastrointestinal histopathologists. Two months after the end of the study, the endoscopists reviewed the still images and video clips, which were randomly sorted. Three endoscopists reviewed the lesions in all patients. At this time, the predictions of histology by each endoscopist for patients who did not undergo endoscopic resection were obtained. Finally, previously collected predictions of histology that were recorded during the procedure were used to analyze the diagnostic accuracy. We classified the histology of the polyps into four categories based on the JNET classification: (1) HP, SSA/P; (2) LGD; (3) HGD, IMC, sSMIC; and (4) dSMIC [14,15]. The endoscopists were asked to predict the histology based on these categories. The diagnostic accuracy was analyzed by comparing the predictions of the endoscopists with the final histology of the polyps. In addition, we calculated the inter-/intra-observer agreement by comparing the results from the case report form and those obtained from reviewing the still images and video clips.
The primary outcome of this study was the comparison of the diagnostic accuracy of the histologic prediction of NBI and i-scan in intermediate-to-large colorectal polyps. The secondary outcome was the estimation of the inter-/intra-observer agreement of the JNET classification between the NBI and i-scan methods.
No previous studies on which the sample size should be based have been published; thus, the number of enrolled patients was determined according to general feasibility. The chi-square test or Fisher’s exact test was used for categorical variables, the results of which are presented as absolute values and percentages. Quantitative data are summarized as means and standard deviations. For comparison, two-sample t-tests or Wilcoxon’s rank sum tests were used according to whether normality assumptions were satisfied or not. The overall accuracy of histologic prediction was analyzed on the basis of the histology results. The inter-/intra-observer agreement was interpreted using the values of κ statistics (slight, ≤0.2; fair, 0.21–0.40; moderate, 0.41–0.60; substantial, 0.61–0.80; and almost perfect, 0.81–1.00) [16]. A two-sided p-value of <0.05 was considered significant. All analyses were performed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA).
The baseline characteristics of the enrolled patients are summarized in Table 1. Sixty-six patients were eligible for this study, all of whom were randomly assigned to the NBI group (n=33) or the i-scan group (n=33). The mean size of the polyps was 18.20±6.98 mm in the NBI group and 19.80±7.13 mm in the i-scan group. The most frequent location of polyps was the ascending colon in both groups (NBI group, n=16, 48.5%; i-scan group, n=12, 36.4%). In addition, no difference was observed in polyp morphology. Most patients underwent endoscopic mucosal resection (NBI group, n=27, 81.8%; i-scan group, n=25, 75.8%) for the treatment of colorectal polyps. The most frequently noted histology in both groups was category 3 (HGD, IMC, and sSMIC) (NBI group, n=17, 51.5%; i-scan group, n=24, 72.7%).
The diagnostic accuracies of NBI versus i-scan are shown in Table 2. The overall diagnostic accuracies in the NBI and i-scan groups were 73.7% (73/99) and 75.8% (75/99), respectively. No statistical significance was observed between the two groups (p=0.744). Furthermore, the detailed diagnostic accuracy of each endoscopist was also documented (Table 2). In addition, we verified their diagnostic yield, including sensitivity, specificity, positive predictive value, negative predictive value, and accuracy. The diagnostic yield was comparable between the NBI and i-scan groups (Table 3).
The JNET classification as applied to NBI and i-scan showed a substantial level of inter-observer agreement (NBI κ-value 0.612, p=0.001 vs. i-scan κ-value 0.662, p=0.002). Additionally, the κ-value of intra-observer agreement ranged from 0.385 to 0.660 for NBI and from 0.364 to 0.741 for i-scan according to each endoscopist (Table 4).
This study demonstrated that NBI and i-scan have comparable efficacy in predicting the histology of intermediate-to-large colorectal polyps. The overall diagnostic accuracies of NBI and i-scan were 73.7% and 75.8%, respectively. In addition, the inter-observer and intra-observer agreements were acceptable among experienced endoscopists. To our knowledge, this is the first randomized study to compare the diagnostic accuracy and agreement between NBI and i-scan for intermediate-to-large colorectal polyps.
Typically, according to clinical practice consensus, adenoma, IMC, and sSMIC are candidates for endoscopic resection. Additionally, dSMIC should be surgically treated because of potential regional lymph node metastasis [17,18]. Therefore, optical estimation of histology is integral in selecting treatment strategies for patients with colorectal polyps. With more accurate predictions, patients can be treated with appropriate procedures, such as endoscopic resection or surgery [19].
In this regard, the use of IEE can more accurately predict histology. IEE offers the advantage of convenience because it does not require additional steps such as the application of dye to the mucosa. To date, the usefulness of NBI for predicting the histology of colorectal polyps has been documented in several reports [20-22]. According to our results, the overall diagnostic accuracy was 73.7% in the NBI group. Recently, a validation study that applied the JNET classification suggested that the optical diagnostic accuracy for colorectal polyps was approximately 90% [23]. This discordance in diagnostic accuracy may be attributed to the different sizes and proportions of polyps with advanced histology. In the previous study, the mean size of the polyps was 13 mm. Conversely, in the present study, the mean size of the polyps was 18 mm. In addition, in this study, the proportion of patients with advanced histology was >51.5% with NBI. The optimal prediction of a JNET 2B lesion is relatively challenging because this category encompasses a wide spectrum of histologic types, including HGD, IMC, and sSMIC. A retrospective study that involved assessment by expert endoscopists showed that the diagnostic accuracy for JNET 2B lesions was 78.1% [15]. Another retrospective study that analyzed still images suggested that the diagnostic accuracy for JNET 2B lesions was 87.4% [24]. Our study documented that the diagnostic accuracy of NBI for JNET 2B lesions was 72.5%.
The i-scan technology, another IEE modality, was recently introduced to clinical practice. To date, several reports have been published on the efficacy of the histologic prediction ability of i-scan [25-27]. As the image enhancement technology of i-scan is similar to that of NBI, we assumed that the JNET classification can be easily applied to i-scan for the prediction of the histology of intermediate-to-large colorectal polyps. Although both NBI and i-scan have shown promising results in the histologic prediction of colorectal polyps, a direct comparison of the prediction by these two methods has not yet been performed in intermediate-to-large colorectal polyps. In the current study, the prediction of the histology of intermediate-to-large colorectal polyps using i-scan was documented to have an overall accuracy of 75.8%. Until now, only two reports have been published on the prediction of small colorectal polyp histology. A prospective study reported an overall accuracy rate of 90.7% for the prediction of diminutive colorectal polyps [12], whereas another randomized study revealed that high-definition colonoscopy with i-scan can predict small, adenomatous polyps with high sensitivity (82%) and specificity (96%) [28].
We estimated the inter-observer and intra-observer agreement for the histologic prediction of colorectal polyps using the JNET classification. The κ-value of the inter-observer agreement among three experienced endoscopists was 0.612 for NBI and 0.662 for i-scan, which indicates acceptable agreement. This result is in concordance with the findings of previous reports. A retrospective analysis of the JNET 2B classification suggested an inter-observer agreement value of 0.743 [14]. In addition, a retrospective validation study of the JNET classification reported a moderate level of inter-observer agreement (κ-value 0.520) [24]. In addition, some studies have explored the inter-observer agreement for histologic prediction using i-scan. A prospective comparative study of NBI and i-scan for the prediction of diminutive colorectal polyps revealed substantial inter-observer agreement (κ-value 0.770) with i-scan [12]. In addition, a prospective study that compared high-definition WLE with i-scan suggested moderate (κ-value 0.463) inter-observer agreement [29]. In our study, the κ-value of the intra-observer agreement ranged from 0.385 to 0.660 for NBI and from 0.364 to 0.741 for i-scan. The small number of cases and the presence of borderline lesions that were classified as JNET 2A/2B may have resulted in a wide-ranging intra-observer agreement.
Several important strengths of our study should be acknowledged. To our knowledge, our study is the first to estimate and compare the diagnostic accuracy of NBI and i-scan in intermediate-to-large colorectal polyps using the JNET classification. As mentioned above, no validated classification of colorectal polyps has been performed with i-scan. The current study showed that the two modalities yielded comparable diagnostic accuracies, particularly when the JNET classification was used for the i-scan mode settings. Additionally, unlike previous retrospective reports in which still images were reviewed, we analyzed the diagnostic accuracy and inter-/intra-observer agreement using a video recording system, which can enhance the reliability and reproducibility of the results.
Despite these strengths, our study also had some limitations. This study was performed by three experienced endoscopists who used NBI and i-scan at a tertiary referral center. In addition, the small sample size might have resulted in misleading diagnostic yields, including positive predictive value, negative predictive value, and accuracy. Therefore, further large-scale trials conducted by endoscopists with different levels of experience are needed to determine the superiority and generalizability of IEE. Moreover, the current study did not alternatively compare each lesion with the different modalities of NBI and i-scan. In addition, the current study was performed using first-generation NBI and an advanced version of i-scan. Thus, a technical gap between the two modalities possibly exists, which may have influenced the results. However, the JNET classification was introduced using first-generation NBI, and consequently, application of the JNET classification to i-scan with this generation of NBI is needed to reduce bias [9].
In conclusion, this study demonstrated that NBI and i-scan have comparable diagnostic accuracies for the histologic prediction of intermediate-to-large colorectal polyps. Furthermore, the inter-observer agreement and intra-observer agreement were acceptable for both NBI and i-scan when the JNET classification was applied.
REFERENCES
1. Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008; 134:1570–1595.

2. Allen JI. Molecular biology of colon polyps and colon cancer. Semin Surg Oncol. 1995; 11:399–405.

3. Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993; 329:1977–1981.
4. Liu HH, Kudo SE, Juch JP. Pit pattern analysis by magnifying chromoendoscopy for the diagnosis of colorectal polyps. J Formos Med Assoc. 2003; 102:178–182.
5. van den Broek FJ, Fockens P, van Eeden S, et al. Endoscopic tri-modal imaging for surveillance in ulcerative colitis: randomised comparison of high-resolution endoscopy and autofluorescence imaging for neoplasia detection; and evaluation of narrow-band imaging for classification of lesions. Gut. 2008; 57:1083–1089.

6. Fu KI, Sano Y, Kato S, et al. Chromoendoscopy using indigo carmine dye spraying with magnifying observation is the most reliable method for differential diagnosis between non-neoplastic and neoplastic colorectal lesions: a prospective study. Endoscopy. 2004; 36:1089–1093.

7. Rex DK, Kahi C, O’Brien M, et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2011; 73:419–422.

8. Hayashi N, Tanaka S, Hewett DG, et al. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the narrow-band imaging international colorectal endoscopic (NICE) classification. Gastrointest Endosc. 2013; 78:625–632.

9. Sano Y, Tanaka S, Kudo SE, et al. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc. 2016; 28:526–533.

10. Kodashima S, Fujishiro M. Novel image-enhanced endoscopy with i-scan technology. World J Gastroenterol. 2010; 16:1043–1049.

11. Hoffman A, Sar F, Goetz M, et al. High definition colonoscopy combined with i-scan is superior in the detection of colorectal neoplasias compared with standard video colonoscopy: a prospective randomized controlled trial. Endoscopy. 2010; 42:827–833.

12. Lee CK, Lee SH, Hwangbo Y. Narrow-band imaging versus i-scan for the real-time histological prediction of diminutive colonic polyps: a prospective comparative study by using the simple unified endoscopic classification. Gastrointest Endosc. 2011; 74:603–609.

13. Tanaka S, Saitoh Y, Matsuda T, et al. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol. 2015; 50:252–260.

14. Sumimoto K, Tanaka S, Shigita K, et al. Diagnostic performance of Japan NBI Expert Team classification for differentiation among noninvasive, superficially invasive, and deeply invasive colorectal neoplasia. Gastrointest Endosc. 2017; 86:700–709.

15. Sumimoto K, Tanaka S, Shigita K, et al. Clinical impact and characteristics of the narrow-band imaging magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Gastrointest Endosc. 2017; 85:816–821.

16. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33:159–174.

17. Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015; 20:207–239.

18. Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015; 27:417–434.

19. Saito Y, Sakamoto T, Nakajima T, Matsuda T. Colorectal ESD: current indications and latest technical advances. Gastrointest Endosc Clin N Am. 2014; 24:245–255.
20. Raghavendra M, Hewett DG, Rex DK. Differentiating adenomas from hyperplastic colorectal polyps: narrow-band imaging can be learned in 20 minutes. Gastrointest Endosc. 2010; 72:572–576.

21. Rex DK. Narrow-band imaging without optical magnification for histologic analysis of colorectal polyps. Gastroenterology. 2009; 136:1174–1181.

22. Mönkemüller K, Zimmermann L. An advanced chromocolonoscopic picture is worth a thousand words, but is it worth the effort? Am J Gastroenterol. 2010; 105:1308–1310.
23. Minoda Y, Ogino H, Chinen T, et al. Objective validity of the Japan Narrow-Band Imaging Expert Team classification system for the differential diagnosis of colorectal polyps. Dig Endosc. 2019; 31:544–551.

24. Komeda Y, Kashida H, Sakurai T, et al. Magnifying narrow band imaging (NBI) for the diagnosis of localized colorectal lesions using the Japan NBI Expert Team (JNET) classification. Oncology. 2017; 93 Suppl 1:49–54.

25. Albrecht H, Nägel A, Tasdelen H, et al. Digital chromoendoscopy with i-scan for in vivo prediction of advanced colorectal neoplasia: a multicenter study. J Clin Gastroenterol. 2016; 50:e91–e94.
26. Klenske E, Zopf S, Neufert C, et al. I-scan optical enhancement for the in vivo prediction of diminutive colorectal polyp histology: results from a prospective three-phased multicentre trial. PLoS One. 2018; 13:e0197520.

27. Glover B, Patel N, Ashrafian H, Teare J. Diagnostic accuracy of i-scan image enhancement for real-time endoscopic diagnosis of small colorectal polyps: a meta-analysis. Therap Adv Gastroenterol. 2018; 11:1756284818814948.

28. Chan JL, Lin L, Feiler M, Wolf AI, Cardona DM, Gellad ZF. Comparative effectiveness of i-SCAN™ and high-definition white light characterizing small colonic polyps. World J Gastroenterol. 2012; 18:5905–5911.
Fig. 1.
Representative images of white-light endoscopy and narrow-band imaging (NBI) obtained from the enrolled patients. (A) Japan NBI Expert Team (JNET) classification 1. (B) JNET classification 2A. (C) JNET classification 2B. (D) JNET classification 3.
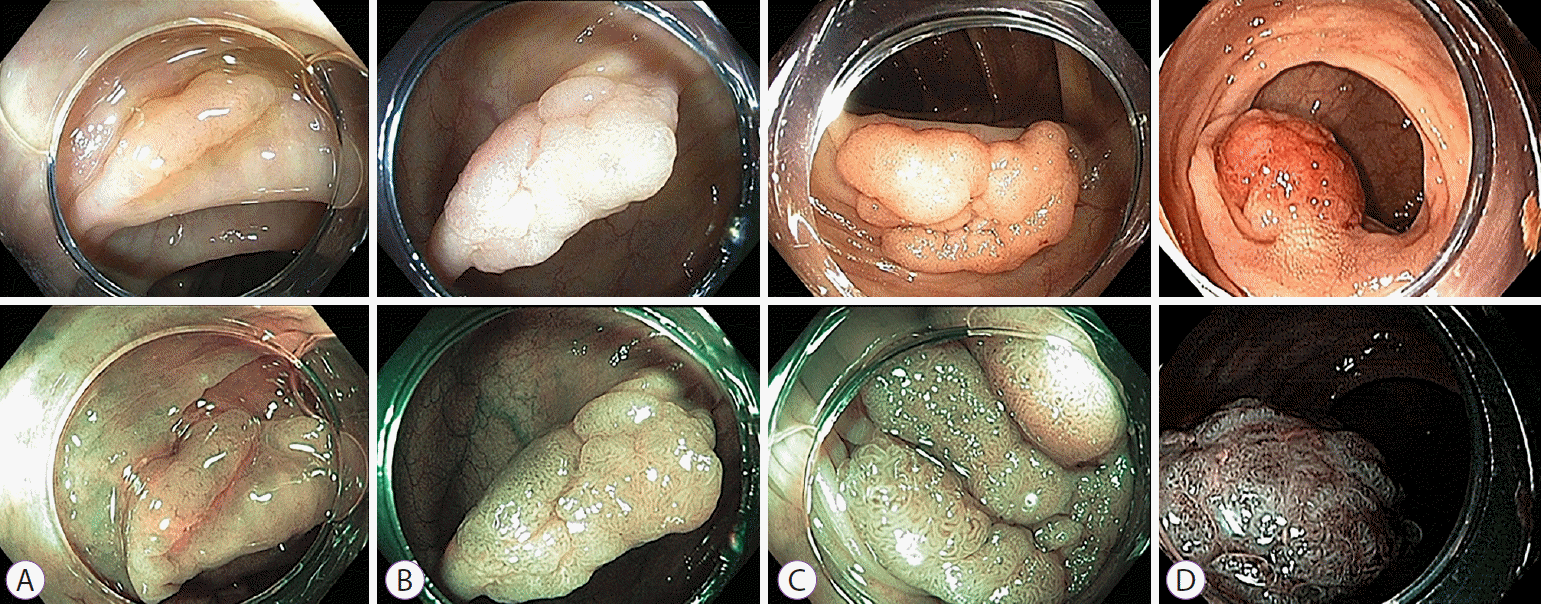
Fig. 2.
Representative images of white-light endoscopy and i-scan obtained from the enrolled patients. (A) Japan Narrow-band Imaging Expert Team (JNET) classification 1. (B) JNET classification 2A. (C) JNET classification 2B. (D) JNET classification 3.
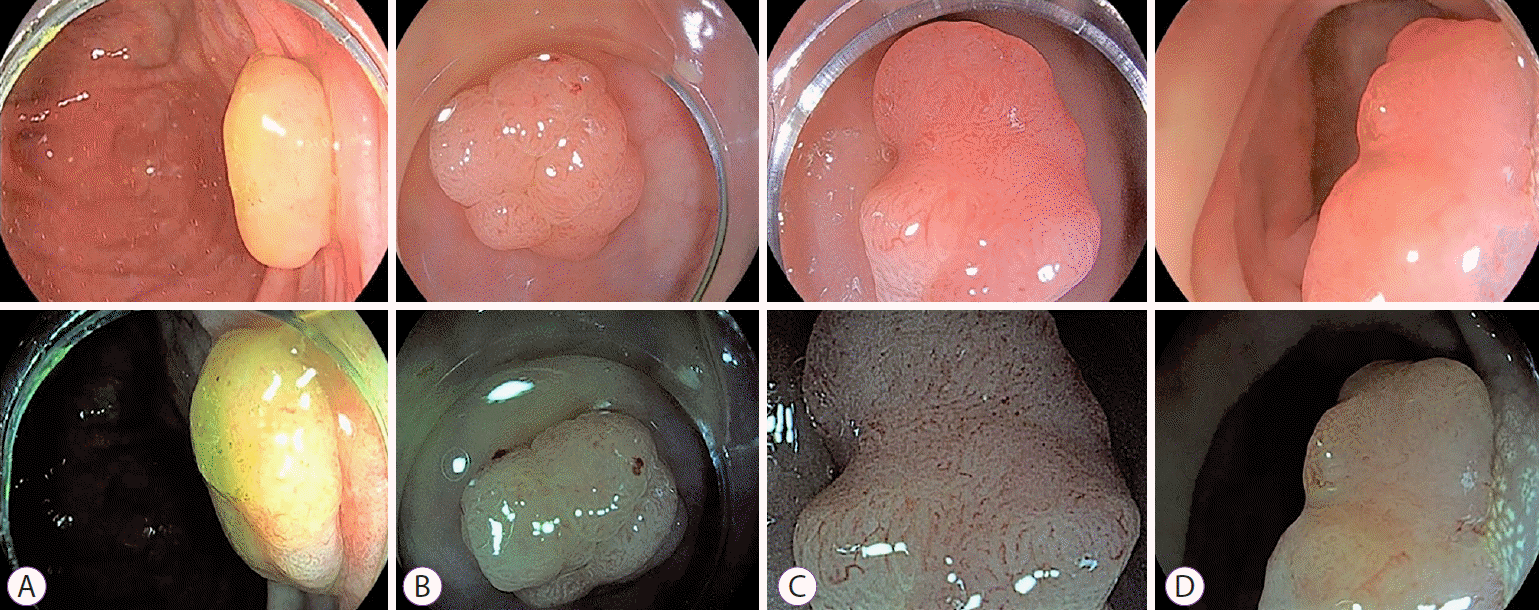
Table 1.
Baseline Characteristics of the Enrolled Patients
| NBI (n=33) | i-scan (n=33) | p-value | |
|---|---|---|---|
| Sex, male | 18 (54.5) | 21 (63.6) | 0.453 |
| Age, yr | 62.50±8.63 | 61.50±11.35 | 0.913 |
| Comorbidity | 0.300 | ||
| None | 15 (45.5) | 22 (66.7) | 0.129 |
| Hypertension | 6 (18.2) | 7 (21.2) | |
| Diabetes mellitus | 4 (12.1) | 2 (6.1) | |
| Othersa) | 8 (24.2) | 2 (6.1) | |
| History of abdominal surgery | 1 (3.0) | 5 (15.2) | 0.087 |
| Lesion size, mm | 18.20±6.98 | 19.80±7.13 | 0.264 |
| Lesion morphology | 0.473 | ||
| Pedunculated | 4 (12.1) | 3 (9.1) | |
| Semi-pedunculated | 9 (27.3) | 12 (36.4) | |
| Sessile | 10 (30.3) | 7 (21.2) | |
| LST-G | 5 (15.2) | 9 (27.3) | |
| LST-NG | 5 (15.2) | 2 (6.1) | |
| Endoscopist who performed the procedures | 0.422 | ||
| Endoscopist 1 | 16 (48.5) | 12 (36.4) | |
| Endoscopist 2 | 8 (24.2) | 7 (21.2) | |
| Endoscopist 3 | 9 (27.3) | 14 (42.4) |
Table 2.
Diagnostic Accuracy of Narrow-Band Imaging and i-scan according to Endoscopist
Table 3.
Diagnostic Yield of Narrow-Band Imaging and i-scan according to Japan Narrow-Band Imaging Expert Team Classification
Table 4.
Inter-/Intra-Observer Agreements of Narrow-Band Imaging and i-scan according to Japan Narrow-Band Imaging Expert Team Classification




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download