1. Suit PF, Petras RE, Bauer TW, Petrini JL Jr. Gastric antral vascular ectasia. A histologic and morphometric study of “the watermelon stomach”. Am J Surg Pathol. 1987; 11:750–757.
2. Gilliam JH 3rd, Geisinger KR, Wu WC, Weidner N, Richter JE. Endoscopic biopsy is diagnostic in gastric antral vascular ectasia. The “watermelon stomach”. Dig Dis Sci. 1989; 34:885–888.
3. Lewis TD, Laufer I, Goodacre RL. Arteriovenous malformation of the stomach. Radiologic and endoscopic features. Am J Dig Dis. 1978; 23:467–471.
4. Jabbari M, Cherry R, Lough JO, Daly DS, Kinnear DG, Goresky CA. Gastric antral vascular ectasia: the watermelon stomach. Gastroenterology. 1984; 87:1165–1170.

5. Petrini JL Jr, Johnston JH. Heat probe treatment for antral vascular ectasia. Gastrointest Endosc. 1989; 35:324–328.

6. Binmoeller KF, Katon RM. Bipolar electrocoagulation for watermelon stomach. Gastrointest Endosc. 1990; 36:399–402.

7. Rose JD. Endoscopic injection of alcohol for bleeding from gastroduodenal vascular anomalies. Br Med J (Clin Res Ed). 1987; 295:93–94.

8. Sargeant IR, Loizou LA, Rampton D, Tulloch M, Bown SG. Laser ablation of upper gastrointestinal vascular ectasias: long term results. Gut. 1993; 34:470–475.

9. Wahab PJ, Mulder CJ, den Hartog G, Thies JE. Argon plasma coagulation in flexible gastrointestinal endoscopy: pilot experiences. Endoscopy. 1997; 29:176–181.

10. Herrera S, Bordas JM, Llach J, et al. The beneficial effects of argon plasma coagulation in the management of different types of gastric vascular ectasia lesions in patients admitted for GI hemorrhage. Gastrointest Endosc. 2008; 68:440–446.

11. Kwan V, Bourke MJ, Williams SJ, et al. Argon plasma coagulation in the management of symptomatic gastrointestinal vascular lesions: experience in 100 consecutive patients with long-term follow-up. Am J Gastroenterol. 2006; 101:58–63.

12. Chaves DM, Sakai P, Oliveira CV, Cheng S, Ishioka S. Watermelon stomach: clinical aspects and treatment with argon plasma coagulation. Arq Gastroenterol. 2006; 43:191–195.

13. Yusoff I, Brennan F, Ormonde D, Laurence B. Argon plasma coagulation for treatment of watermelon stomach. Endoscopy. 2002; 34:407–410.

14. Boltin D, Gingold-Belfer R, Lichtenstein L, Levi Z, Niv Y. Long-term treatment outcome of patients with gastric vascular ectasia treated with argon plasma coagulation. Eur J Gastroenterol Hepatol. 2014; 26:588–593.

15. Nakamura S, Mitsunaga A, Konishi H, Oi I, Shiratori K, Suzuki S. Long-term follow up of gastric antral vascular ectasia treated by argon plasma coagulation. Dig Endosc. 2006; 18:128–133.

16. Garg S, Aslam B, Nickl N. Endoscopic resolution and recurrence of gastric antral vascular ectasia after serial treatment with argon plasma coagulation. World J Gastrointest Endosc. 2017; 9:263–266.

17. Jana T, Thosani N, Fallon MB, Dupont AW, Ertan A. Radiofrequency ablation for treatment of refractory gastric antral vascular ectasia (with video). Endosc Int Open. 2015; 3:E125–E127.

18. Magee C, Lipman G, Alzoubaidi D, et al. Radiofrequency ablation for patients with refractory symptomatic anaemia secondary to gastric antral vascular ectasia. United European Gastroenterol J. 2019; 7:217–224.

19. Wells CD, Harrison ME, Gurudu SR, et al. Treatment of gastric antral vascular ectasia (watermelon stomach) with endoscopic band ligation. Gastrointest Endosc. 2008; 68:231–236.

20. Keohane J, Berro W, Harewood GC, Murray FE, Patchett SE. Band ligation of gastric antral vascular ectasia is a safe and effective endoscopic treatment. Dig Endosc. 2013; 25:392–396.

21. Zepeda-Gómez S, Sultanian R, Teshima C, Sandha G, Van Zanten S, Montano-Loza AJ. Gastric antral vascular ectasia: a prospective study of treatment with endoscopic band ligation. Endoscopy. 2015; 47:538–540.

22. Sato T, Yamazaki K, Akaike J. Endoscopic band ligation versus argon plasma coagulation for gastric antral vascular ectasia associated with liver diseases. Dig Endosc. 2012; 24:237–242.

23. Eccles J, Falk V, Montano-Loza AJ, Zepeda-Gómez S. Long-term follow-up in patients with gastric antral vascular ectasia (GAVE) after treatment with endoscopic band ligation (EBL). Endosc Int Open. 2019; 7:E1624–E1629.

24. Polski JM, Brunt EM, Saeed ZA. Chronology of histological changes after band ligation of esophageal varices in humans. Endoscopy. 2001; 33:443–447.

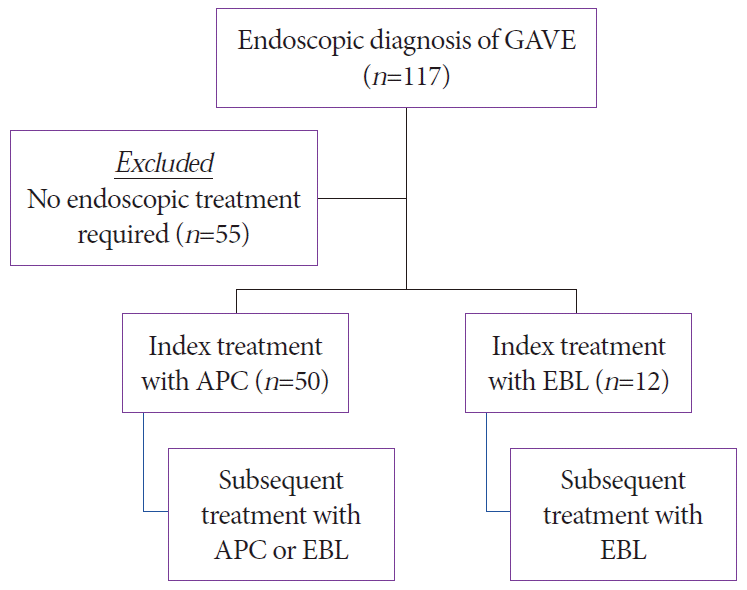




 PDF
PDF Citation
Citation Print
Print



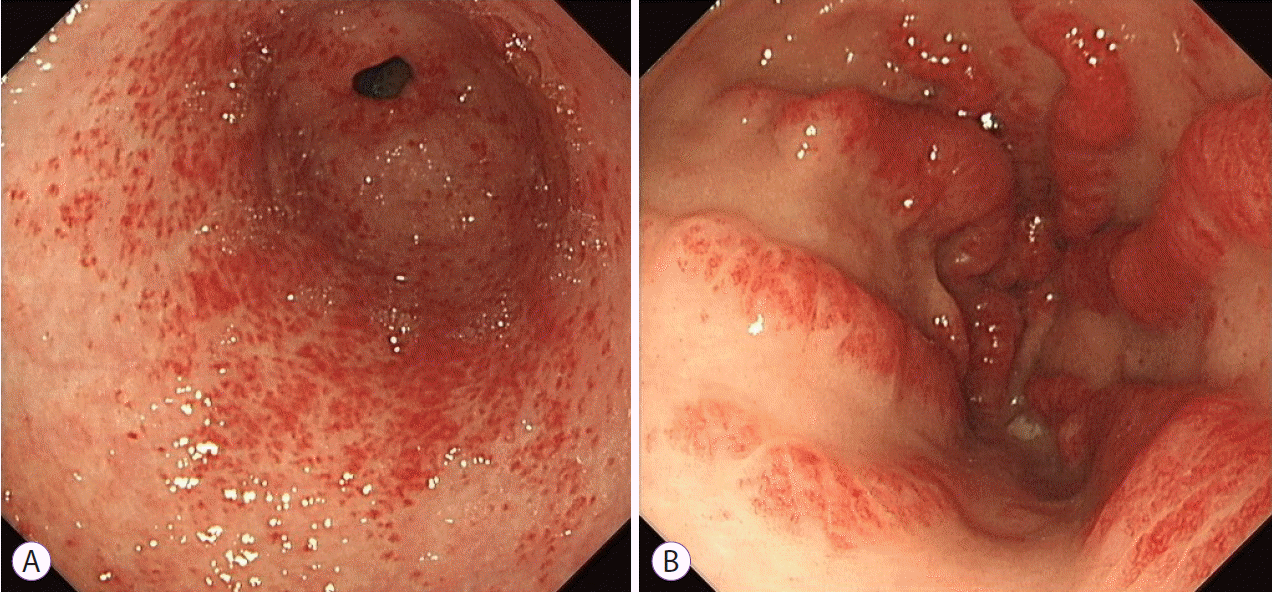
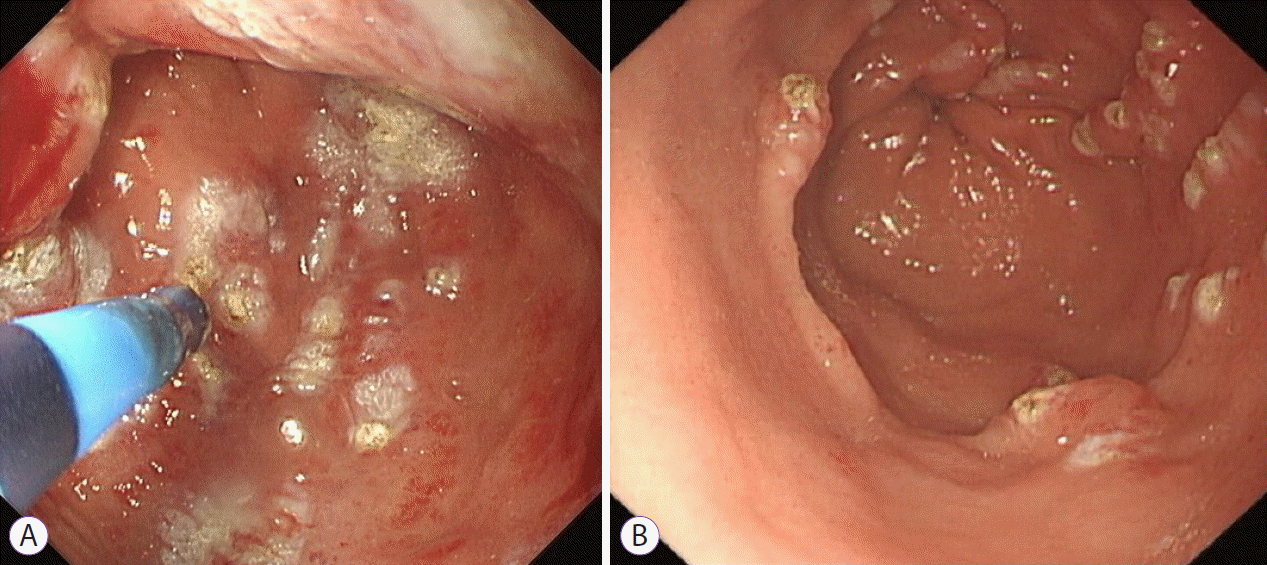
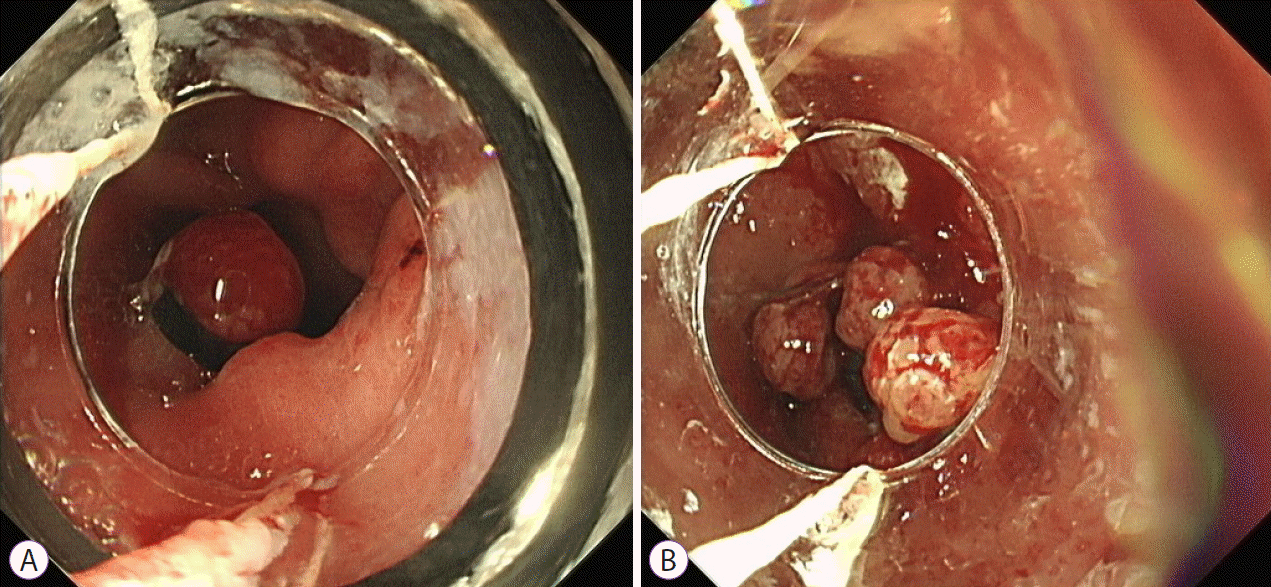
 XML Download
XML Download