Abstract
Background/Aims
The management of walled-off necrosis (WON) has undergone a paradigm shift from surgical to nonsurgical modalities. Real-world data on the management of symptomatic WON are scarce.
Methods
Prospectively collected data of symptomatic WON cases were retrospectively evaluated. The treatment modalities used were medical management alone, percutaneous catheter drainage (PCD) or endoscopic drainage (ED), or a combination of PCD and ED. We compared clinical outcome among these modalities.
Results
A total of 264 patients were evaluated. The most common indications for drainage were pain and fever. Of the patients, 28% was treated with medical therapy alone, 31% with ED, 37% with PCD, and 4% with a combined approach. Technical success and clinical success were achieved in 93% and 91% of patients in the endoscopic arm and in 90% and 81% patients in the PCD arm, respectively (p=0.0004 for clinical success). Lower rates of complications (7% vs. 22%, p=0.005), readmission (20% vs. 34%, p=0.04), and mortality (4% vs. 19%, p=0.0012), and shorter hospital stay (13 days vs. 19 days, p=0.0018) were observed in the endoscopic group than in the PCD group.
Go to : 
Acute pancreatitis (AP) is a common disease entity in gastroenterology practice. Overall, 85% of patients have interstitial pancreatitis and 15% (range, 4–47%) have necrotizing pancreatitis, which is associated with a higher mortality [1]. The major cause of death, besides early organ failure, is secondary infection of pancreatic or peripancreatic necrotic tissue, leading to sepsis and multiple organ failure [2]. According to the revised Atlanta classification, these necrotic collections are termed walled-off necrosis (WON), occurring 4 weeks after the onset of the disease [3]. WON requires drainage if it becomes symptomatic or infected [4,5]. The treatment modalities of WON drainage are surgical [6], endoscopic [7], and percutaneous [8], with further step-up to minimally invasive retroperitoneal necrosectomy [9] if required. Surgical open necrosectomy has been shown to be inferior to the step-up approach, as it leads to more complications [10]. In fact, a considerable number of patients do not require surgery when treated using a step-up approach [11].
Endoscopic transgastric drainage has also been shown to be better than surgical necrosectomy, with fewer complications (20% vs. 80%) [12]. In the recently published TENSION trial, endoscopic drainage was shown to be noninferior to the percutaneous step-up approach in resolving WON, with reduced rates of fistula formation and shorter hospital stay [13]. Thus, the current evidence favors the step-up and endoscopic approach for WON drainage over the surgical approach, with a shorter hospital stay and reduced fistula formation in patients undergoing endoscopic drainage.
However, all these previous studies were rigorously conducted trials and there is a lack of real-world data on the efficacy and safety of the various modalities used for WON drainage. This study presents a real-world scenario of the management of WON at a tertiary care center in India.
Go to : 
This was a prospective observational study conducted at a tertiary care center in North India (Department of Gastroenterology, Govind Ballabh Pant Institute of Postgraduate Medical Education and Research, New Delhi, India). The study was conducted from January 1, 2016 to December 31, 2019. All patients who were admitted to the hospital and underwent drainage for symptomatic WON were included in the study. WON was defined according to the revised Atlanta classification [3]. Drainage was performed either percutaneously or under endoscopic guidance, or through a combination of percutaneous catheter drainage (PCD) and endoscopic drainage whenever needed.
Combined presence of pain, elevated serum amylase/lipase level, and/or radiologic evidence of pancreatitis.
According to the Atlanta 2012 classification, it is a sequel of acute necrotizing pancreatitis of >4 weeks’ duration showing a heterogeneous encapsulated collection on contrast-enhanced computed tomography (CECT) of the abdomen.
Occurrence of WON with any of the following symptoms and signs:
• Fever, tachycardia (presence of infection)
• Persistent abdominal pain requiring parenteral analgesics
• Features of luminal or biliary obstruction resulting from external compression.
All patients with symptomatic WON were treated as inpatients. They were managed, according to the presence of clinical symptoms, with intravenous fluids and analgesics. The patients were administered 1,500–2,000 kcal/day and 50–60 g protein/day of enteral nutrition whenever possible. Patients suspected of having an infection because of the presence of fever or elevated leukocyte counts were treated with intravenous broad-spectrum antibiotics. After the initial stabilization, the patients underwent cross-sectional imaging to determine the size and location of the collection and the presence of collaterals. The decision on the mode of drainage was made on the basis of imaging findings and the patients’ clinical condition. If the WON was in close relation to the gastrointestinal tract and the patient was stable enough to undergo an endoscopic procedure, endoscopic drainage was performed. Otherwise, PCD was performed under ultrasonographic or computed tomography guidance, and a pigtail catheter was placed. The patients were observed for any procedure-related complications. In the case of infection, the patients were administered intravenous antibiotics until 48 hr after becoming afebrile. Any change in antibiotic therapy was guided by the culture results of the aspirated fluid.
All patients in this group underwent endoscopic ultrasound (EUS) in the left lateral position (linear echo-endoscope; Olympus, Tokyo, Japan). The collections were localized, and the transgastric or transduodenal approach was selected depending on the location. The avascular site was marked and punctured using a 19-G fine-needle aspiration needle, and fluid was aspirated to confirm the position. A 0.025-in VisiGlide guidewire with a distal hydrophilic tip was passed into the cavity. The puncture site was dilated with a 6-Fr cystotome (Shaili Endoscopy, Gujarat, India) followed by a 10-mm controlled radial expansion biliary dilator. Either two 10-Fr, 7-cm double-pigtail (DPT) plastic stents or a single 30 mm × 16 mm self-expanding metallic stent (SEMS, NAGITM; Taewoong Medical, Goyang, Korea) was placed, with one end inside the cavity and the other in the stomach/duodenum. The choice of stent to be placed was based on magnetic resonance imaging (MRI) assessment (>30% debris for SEMS) and/or endosonographic evaluation of the collection for the amount of debris and the nature of aspirated fluid. The patients were allowed oral intake at 6 hr after the procedure. If there was no clinical improvement in 72 hr, forward-viewing endoscopy was performed to look for the stent. If the stent was blocked, then the scope was introduced inside the cavity through the stent and necrosectomy was performed. If there was clinical improvement in 72 hr, the patients were followed up every week as outpatients. If the clinical improvement continued, the patients were regularly followed up for 3 months and the stent was removed according to the resolution of the collection. If clinical deterioration was observed, the patients underwent upper gastrointestinal endoscopy and necrosectomy, as earlier described. If there was no improvement after four attempts, the patients underwent surgery.
A percutaneous drain (12-Fr or more) was placed in the collection. The preferred route was through the left retroperitoneum. If this was not possible, a transperitoneal route was chosen. The right retroperitoneal route was only allowed when it could be safely applied. Drains were kept open by aspiration using a 5–10 mL sterile syringe without flushing, once every 8 hr. The patient was monitored closely after drain placement. Repeat imaging was performed as and when required. Drains were upgraded up to a 16-Fr size, as and when required. If the position of the drain was inadequate, then either it was repositioned or a second drain was placed in the collection. The patients were followed up clinically and with imaging (mostly ultrasonography or CECT if required), and drains were removed after complete recovery.
We collected data on history, anthropometry, general/systemic examination, imaging (at baseline and after the completion of 3 months, and in between as and when required), CECT to confirm the collection, and MRI to quantify the necrotic material.
We compared the treatment modalities in terms of technical success, clinical success, mortality, readmissions, complications, and length of hospital stay.
Categorical variables are described using absolute and relative frequencies, and continuous variables are described as mean and standard deviation (SD), or median and range, whenever appropriate. Categorical variables were compared using the chi-square/Fisher’s exact test, whereas the t-test was used for comparison of quantitative data. All reported p-values are two-sided, and p<0.05 was considered statistically significant. All data were arranged, processed, and analyzed with IBM SPSS Statistics for Windows, version 23.0. (IBM Co., Armonk, NY, USA).
Go to : 
During the study period, 264 patients treated for symptomatic WON were analyzed. The majority were men (n=195, 74%). The mean age was 37.66 (SD, 14.41) years.
Overall, the most common etiologies were excessive alcohol consumption (n=115, 44%) and biliary i.e., gall stones, microlithiasis (n=89, 34%).
Pain was the most common reason for admission and was present in 253 patients (96%), followed by fever, which was seen in 140 (53%) patients. Other symptoms included jaundice and features of partial or complete luminal obstruction (gastric outlet obstruction), which were present in 16 (6%) and 14 (5%) patients, respectively.
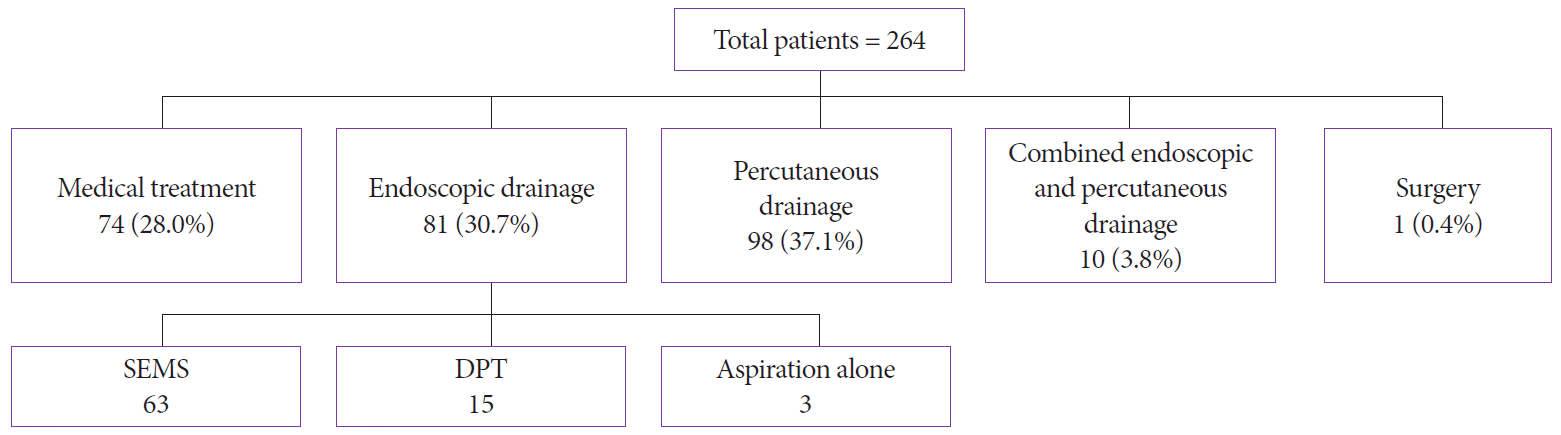
After analyzing the treatment, we found that 74 (28%) patients were treated with medical therapy alone (i.e., nutrition therapy, antibiotics, and fluids), whereas 81 (31%) patients underwent endoscopic drainage (among whom 63 patients underwent SEMS placement and 15 patients underwent DPT plastic stent placement) and 3 patients underwent endoscopic aspiration owing to failure of stent placement. In this arm, 14 patients (17.28%) needed endoscopic necrosectomy. The technical success rate of endoscopic drainage was 93% (n=78). In total, 98 (37%) patients had a percutaneous drain placed. Meanwhile, 10 (4%) patients were treated with combined management (i.e., both PCD and endoscopic drainage). One patient underwent surgery. The overall mortality rate was 13% (n=34) (Fig. 1).
The baseline characteristics of the two groups were similar (Table 1). The baseline hemoglobin, albumin, and creatinine levels were also comparable. Eleven (13.58%) patients from the endoscopic group and 19 (19.38%) patients from the percutaneous group had severe pancreatitis according to the Atlanta 2012 classification (p=0.30).
Baseline Characteristics of Study Population in Two Treatment Arms
Technical and clinical success was achieved in 77 (93%) and 74 (91%) patients in the endoscopic arm and in 88 (90%) and 79 (81%) patients in the percutaneous arm, respectively. There was a statistically significant difference (Table 2) in clinical success, with endoscopic drainage showing better results. Four patients in whom endoscopic drainage failed were managed with EUS-guided single-time aspiration and culture-based antibiotics, whereas 10 patients with failed PCD were medically treated with antibiotics and nutrition. In addition, readmission owing to either the reappearance or nonresolution of symptoms was observed in 16 (20%) and 33 (34%) patients in the endoscopic drainage and PCD arms, respectively (p=0.04) (Table 2).
Comparison of Outcomes in Two Groups
The total number of complications in the endoscopic arm was 6 (7%) and that in the percutaneous arm was 22 (22%) (p=0.005). However, all six complications in the endoscopic arm were major (two bleeding cases, three cases of stent migration requiring intervention, and one case of cystocolic fistula), whereas 11 (11%) of the total 22 complications in the percutaneous arm were major (six bleeding cases, four cases of cystocolic fistulas, and one case of WON rupture in the peritoneum). The remaining 11 complications were minor (nine cases of cystocutaneous fistulas that spontaneously healed in 4 weeks and two cases of Clostridium difficile-associated diarrhea) treated on an outpatient basis.
Overall, three (4%) patients in the endoscopic arm died, whereas 19 (19%) patients in the PCD arm died, with a statistically significant difference (p<0.05) between the two groups.
We also compared the length of hospital stay between the two treatment arms. The average duration of hospitalization was 13 (±11) days in the endoscopic arm and 19 (±14) days in the percutaneous arm, and the difference (+6 days) was statistically significant (p<0.05) (Table 2).
Go to : 
To the best of our knowledge, this is one of the first real-world studies to illustrate a trend toward the exclusive use of minimally invasive therapies for pancreatic walled-off collections (WON). In fact, only one patient underwent surgery. Our study shows that minimally invasive therapies are acceptable treatment modalities in patients with symptomatic WON and have supplanted surgical necrosectomy for the management of WON. Another highlight of this study is that it shows a clear benefit of the endoscopic approach over the percutaneous approach for the management of WON. In addition, this study shows that although the technical success rate of endoscopic drainage is equal to that of PCD, endoscopic drainage is better in terms of overall clinical success, length of hospital stay, number of readmissions, number of total complications, and survival (p<0.05 for each of these outcomes).
WON constitutes 1–9% of all AP complications and remains a life-threatening entity, which can be treated through early detection and application of the indicated therapeutic measures [14]. Most studies show that nearly 50% of patients are conservatively managed [15]. In the current study, of 264 WON patients, 180 (72%) required intervention, which is almost 20% more than what is mentioned in the literature. This increased rate of intervention indicated a referral bias, as our hospital is a tertiary care referral center for patients with WON.
Similar to previous studies [16-18], our study also showed that the most common indications for intervention were pain and fever in patients with WON, accounting for almost 90–95% of the cases.
As stated above, the last few decades have seen a paradigm shift in the management of pancreatic fluid collections [19,20]. PCD is one of the first minimally invasive techniques employed, with gradual improvisation in techniques with improved outcomes. Freeny et al. was the first to perform PCD in 34 patients and had a success rate of 47% [8]. Their study was followed by many other studies that achieved a success rate of up to 90% [14,21-25]. In our study, a total of 98 patients were treated with PCD. Technical success was achieved in 88 (90%) patients and clinical success in 79 (81%) patients, which are similar to the findings of the majority of recent studies [19,24,25].
In our study, the complication rate was 22% (n=22), in the form of external or internal fistulas and bleeding in the PCD arm. All cutaneous fistulas spontaneously healed. However, the most dreaded complication was bleeding from the WON cavity either due to pseudo-aneurysm rupture or vascular injury due to sepsis or trauma. In the published literature, the most common complications of PCD are gastrointestinal fistulas, which occur at a rate of up to 20% and do not require any specific treatment [13,26-28].
The most important advantage of PCD is the ease of performance and the possibility of access to almost any region of the abdominal cavity. This procedure can also be performed in remote areas, with a small learning curve. Therefore, it will always have a place in the management of WON, especially for patients whose collections are not endoscopically accessible or who are too sick to undergo endoscopic drainage.
Since the first endoscopic treatment of WON performed by Baron in 1996, this method has rapidly evolved [29]. The initial decades of the 21st century have seen the evolution from simple endoscopic drainage to the use of EUS, which prevents failure and procedure-related vascular complications, and from plastic stents to the use of wide-bore lumen apposing metallic stents, which allow direct necrosectomy. These newer approaches are successful in approximately 90%, compared with 50% in standard endoscopic drainage [7,30-35]. Varadarajulu et al. described a new EUS-based approach with an associated success rate of 91.7% [17].
The present study included 81 patients treated with endoscopic drainage. EUS-guided metallic stents were placed in almost 78% (63/81), and DPT plastic stents were placed in the remaining 15 patients, with overall technical success and clinical success of 93% and 91%, respectively, similar to the findings of Varadarajulu et al. [17].
All complications in the endoscopic arm were major. Two patients in this arm required surgical intervention. One of these patients had migration of the stent into the small bowel, leading to recurrent episodes of pain and one episode of subacute intestinal obstruction. We attempted to access the stent using endoscopy; however, we failed to remove the stent. Finally, the patient underwent surgical intervention for stent removal.
We hypothesized that endoscopic drainage adds to enteral nutrition by draining pus into the gastrointestinal tract, which undergoes proteolysis and absorption, leading to accelerated recovery by supplementing nutrition, which may be the reason for the decreased period of hospitalization in the endoscopic drainage group. As severe AP affects mostly young adults in the productive age group, and the length of hospital stay is known to be inversely related to productivity, it places an additional burden on a household level. This further highlights the role of the endoscopic approach in PCD.
The PANTHER [28] and TENSION [13] trials also observed shorter hospitalization and lesser fistula formation in the endoscopic step-up arm. When we analyzed our results in comparison with the Dutch (TENSION) trial, we found a few differences. The first major difference is that we used a widebore SEMS in almost 80% of the endoscopic treatment arm, whereas DPT stents were used for drainage in the majority of patients in the TENSION trial. Second, we included patients with symptomatic WON, whereas the Dutch trial included patients with infected necrotic collection (including patients at <4 weeks of disease onset), which explains the greater mortality in the TENSION trial, as patients with infected collection were sicker.
The major strength of our study is that it demonstrates the outcome of minimally invasive therapy in patients with WON who are not part of a trial or proof-of-concept study, and thus represents a real-world scenario. The fact that we achieved technical and clinical rates comparable to randomized trials further proves the efficacy and safety of these therapies. The limitations are that we did not assess the quality of life of patients in both arms and a comparative cost analysis was not performed because treatment is free of cost at our center.
In conclusion, endoscopic drainage for the management of WON is better than PCD in terms of efficacy and survival, and is associated with fewer complications, shorter hospitalization, and fewer readmissions.
Go to : 
REFERENCES
1. Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006; 101:2379–2400.

2. Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006; 354:2142–2150.
3. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013; 62:102–111.

4. Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013; 108:1400–1415. 1416.

5. Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013; 13(4 Suppl 2):e1–e15.
6. Beger HG, Büchler M, Bittner R, Block S, Nevalainen T, Roscher R. Necrosectomy and postoperative local lavage in necrotizing pancreatitis. Br J Surg. 1988; 75:207–212.

7. Papachristou GI, Takahashi N, Chahal P, Sarr MG, Baron TH. Peroral endoscopic drainage/debridement of walled-off pancreatic necrosis. Ann Surg. 2007; 245:943–951.

8. Freeny PC, Hauptmann E, Althaus SJ, Traverso LW, Sinanan M. Percutaneous CT-guided catheter drainage of infected acute necrotizing pancreatitis: techniques and results. AJR Am J Roentgenol. 1998; 170:969–975.

9. van Santvoort HC, Besselink MG, Horvath KD, et al. Videoscopic assisted retroperitoneal debridement in infected necrotizing pancreatitis. HPB (Oxford). 2007; 9:156–159.

10. van Santvoort HC, Besselink MG, Bakker OJ, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010; 362:1491–1502.
11. van Baal MC, van Santvoort HC, Bollen TL, Bakker OJ, Besselink MG, Gooszen HG. Systematic review of percutaneous catheter drainage as primary treatment for necrotizing pancreatitis. Br J Surg. 2011; 98:18–27.

12. Bakker OJ, van Santvoort HC, van Brunschot S, et al. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA. 2012; 307:1053–1061.

13. van Brunschot S, van Grinsven J, van Santvoort HC, et al. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: a multicentre randomised trial. Lancet. 2018; 391:51–58.
14. Chang YC, Tsai HM, Lin XZ, Chang CH, Chuang JP. No debridement is necessary for symptomatic or infected acute necrotizing pancreatitis: delayed, mini-retroperitoneal drainage for acute necrotizing pancreatitis without debridement and irrigation. Dig Dis Sci. 2006; 51:1388–1395.

15. Wroński M, Cebulski W, Pawłowski W, Krasnodębski IW, Słodkowski M. Walled-off necrosis: safety of watchful waiting. Dig Dis Sci. 2015; 60:1081–1086.

16. Ramia JM, de la Plaza R, Quiñones-Sampedro JE, Ramiro C, Veguillas P, García-Parreño J. Walled-off pancreatic necrosis. Neth J Med. 2012; 70:168–171.
17. Varadarajulu S, Phadnis MA, Christein JD, Wilcox CM. Multiple transluminal gateway technique for EUS-guided drainage of symptomatic walled-off pancreatic necrosis. Gastrointest Endosc. 2011; 74:74–80.

18. Gluck M, Ross A, Irani S, et al. Endoscopic and percutaneous drainage of symptomatic walled-off pancreatic necrosis reduces hospital stay and radiographic resources. Clin Gastroenterol Hepatol. 2010; 8:1083–1088.

19. Chang YC. Is necrosectomy obsolete for infected necrotizing pancreatitis? Is a paradigm shift needed? World J Gastroenterol. 2014; 20:16925–16934.

20. Yip HC, Teoh AYB. Endoscopic management of peri-pancreatic fluid collections. Gut Liver. 2017; 11:604–611.

21. Bruennler T, Langgartner J, Lang S, et al. Outcome of patients with acute, necrotizing pancreatitis requiring drainage-does drainage size matter? World J Gastroenterol. 2008; 14:725–730.

22. Navalho M, Pires F, Duarte A, Gonçalves A, Alexandrino P, Távora I. Percutaneous drainage of infected pancreatic fluid collections in critically ill patients: correlation with C-reactive protein values. Clin Imaging. 2006; 30:114–119.

23. Mortelé KJ, Girshman J, Szejnfeld D, et al. CT-guided percutaneous catheter drainage of acute necrotizing pancreatitis: clinical experience and observations in patients with sterile and infected necrosis. AJR Am J Roentgenol. 2009; 192:110–116.

24. Sleeman D, Levi DM, Cheung MC, et al. Percutaneous lavage as primary treatment for infected pancreatic necrosis. J Am Coll Surg. 2011; 212:748–752. discussion 752-754.

25. Zerem E, Imamović G, Sušić A, Haračić B. Step-up approach to infected necrotising pancreatitis: a 20-year experience of percutaneous drainage in a single centre. Dig Liver Dis. 2011; 43:478–483.

26. Wroński M, Cebulski W, Słodkowski M, Krasnodębski IW. Minimally invasive treatment of infected pancreatic necrosis. Prz Gastroenterol. 2014; 9:317–324.

27. Jagielski M, Smoczyński M, Jabłońska A, Marek I, Dubowik M, Adrych K. The role of endoscopic ultrasonography in endoscopic debridement of walled-off pancreatic necrosis--a single center experience. Pancreatology. 2015; 15:503–507.
28. Besselink MG, van Santvoort HC, Nieuwenhuijs VB, et al. Minimally invasive ‘step-up approach’ versus maximal necrosectomy in patients with acute necrotising pancreatitis (PANTER trial): design and rationale of a randomised controlled multicenter trial [ISRCTN13975868]. BMC Surg. 2006; 6:6.

29. Baron TH, Kozarek RA. Endotherapy for organized pancreatic necrosis: perspectives after 20 years. Clin Gastroenterol Hepatol. 2012; 10:1202–1207.

30. Gardner TB, Coelho-Prabhu N, Gordon SR, et al. Direct endoscopic necrosectomy for the treatment of walled-off pancreatic necrosis: results from a multicenter U.S. series. Gastrointest Endosc. 2011; 73:718–726.

31. Seewald S, Groth S, Omar S, et al. Aggressive endoscopic therapy for pancreatic necrosis and pancreatic abscess: a new safe and effective treatment algorithm (videos). Gastrointest Endosc. 2005; 62:92–100.

32. Voermans RP, Veldkamp MC, Rauws EA, Bruno MJ, Fockens P. Endoscopic transmural debridement of symptomatic organized pancreatic necrosis (with videos). Gastrointest Endosc. 2007; 66:909–916.

33. Coelho D, Ardengh JC, Eulálio JM, Manso JE, Mönkemüller K, Coelho JF. Management of infected and sterile pancreatic necrosis by programmed endoscopic necrosectomy. Dig Dis. 2008; 26:364–369.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download