1. Goudra B, Nuzat A, Singh PM, Gouda GB, Carlin A, Manjunath AK. Cardiac arrests in patients undergoing gastrointestinal endoscopy: a retrospective analysis of 73,029 procedures. Saudi J Gastroenterol. 2015; 21:400–411.

2. Goudra B, Nuzat A, Singh PM, Borle A, Carlin A, Gouda G. Association between type of sedation and the adverse events associated with gastrointestinal endoscopy: an analysis of 5 years’ data from a tertiary center in the USA. Clin Endosc. 2017; 50:161–169.

3. Gleason JM, Christian BR, Barton ED. Nasal cannula apneic oxygenation prevents desaturation during endotracheal intubation: an integrative literature review. West J Emerg Med. 2018; 19:403–411.

4. Bielawska B, Hookey LC, Sutradhar R, et al. Anesthesia assistance in outpatient colonoscopy and risk of aspiration pneumonia, bowel perforation, and splenic injury. Gastroenterology. 2018; 154:77–85.e3.

5. Wernli KJ, Brenner AT, Rutter CM, Inadomi JM. Risks associated with anesthesia services during colonoscopy. Gastroenterology. 2016; 150:888–894. quiz e18.

6. Goudra B, Singh PM, Gouda G, Borle A, Carlin A, Yadwad A. Propofol and non-propofol based sedation for outpatient colonoscopy-prospective comparison of depth of sedation using an EEG based SEDLine monitor. J Clin Monit Comput. 2016; 30:551–557.

7. Sahay N, Sharma S, Bhadani UK, Sinha C, Kumar A, Ranjan A. Effect of nasal oxygen supplementation during apnoea of intubation on arterial oxygen levels: a prospective randomised controlled trial. Indian J Anaesth. 2017; 61:897–902.

8. Nishimura M. High-flow nasal cannula oxygen therapy devices. Respir Care. 2019; 64:735–742.

9. Zhu Y, Yin H, Zhang R, Ye X, Wei J. High-flow nasal cannula oxygen therapy versus conventional oxygen therapy in patients after planned extubation: a systematic review and meta-analysis. Crit Care. 2019; 23:180.

10. Parke RL, Eccleston ML, McGuinness SP. The effects of flow on airway pressure during nasal high-flow oxygen therapy. Respir Care. 2011; 56:1151–1155.

11. Lin Y, Zhang X, Li L, et al. High-flow nasal cannula oxygen therapy and hypoxia during gastroscopy with propofol sedation: a randomized multicenter clinical trial. Gastrointest Endosc. 2019; 90:591–601.

17. Gedeon M, Gomes S, Roy K, Duclos-Miller P, Rose JS. Use of noninvasive positive pressure ventilation in patients with severe obesity undergoing esophagogastroduodenoscopy: a randomized controlled trial. Surg Obes Relat Dis. 2019; 15:1589–1594.

18. Dimou F, Huynh S, Dakin G, et al. Nasal positive pressure with the SuperNO2VATM device decreases sedation-related hypoxemia during pre-bariatric surgery EGD. Surg Endosc. 2019; 33:3828–3832.
19. Bai Y, Xu Z, Chandrashekar M, et al. Comparison of a simplified nasal continuous positive airways pressure device with nasal cannula in obese patients undergoing colonoscopy during deep sedation: a randomised clinical trial. Eur J Anaesthesiol. 2019; 36:633–640.
20. Goudra BG, Singh PM, Sinha AC. Outpatient endoscopic retrograde cholangiopancreatography: safety and efficacy of anesthetic management with a natural airway in 653 consecutive procedures. Saudi J Anaesth. 2013; 7:259–265.

21. Goudra BG, Singh PM, Penugonda LC, Speck RM, Sinha AC. Significantly reduced hypoxemic events in morbidly obese patients undergoing gastrointestinal endoscopy: predictors and practice effect. J Anaesthesiol Clin Pharmacol. 2014; 30:71–77.

22. Goudra BG, Penugonda LC, Sinha A. A novel way of anesthetizing and maintaining airway/ventilation in an ultra-morbidly obese patient presenting for upper GI endoscopy. J Clin Anesth. 2012; 24:604–605.

23. Qin Y, Li LZ, Zhang XQ, et al. Supraglottic jet oxygenation and ventilation enhances oxygenation during upper gastrointestinal endoscopy in patients sedated with propofol: a randomized multicentre clinical trial. Br J Anaesth. 2017; 119:158–166.

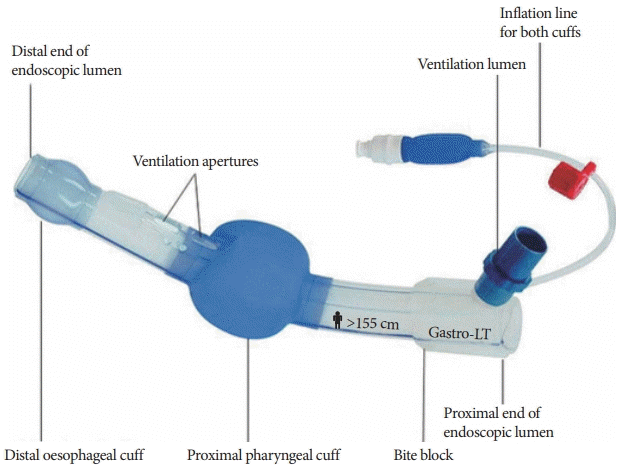
25. Gaitini LA, Lavi A, Stermer E, Charco Mora P, Pott LM, Vaida SJ. Gastro-laryngeal tube for endoscopic retrograde cholangiopancreatography: a preliminary report. Anaesthesia. 2010; 65:1114–1118.

26. Fabbri C, Luigiano C, Cennamo V, et al. The Gastro-laryngeal tube for interventional endoscopic biliopancreatic procedures in anesthetized patients. Endoscopy. 2012; 44:1051–1054.

27. Davis J, Sreevastava DK, Dwivedi D, Gadgi S, Sud S, Dudeja P. A comparison of stress response between insertion of gastro-laryngeal tube and endotracheal intubation in patients undergoing upper gastrointestinal endoscopic procedures for endoscopic retrograde cholangiopancreatography. Anesth Essays Res. 2019; 13:13–18.

28. Barnett SR, Berzin T, Sanaka S, Pleskow D, Sawhney M, Chuttani R. Deep sedation without intubation for ERCP is appropriate in healthier, non-obese patients. Dig Dis Sci. 2013; 58:3287–3292.

29. Terblanche NCS, Middleton C, Choi-Lundberg DL, Skinner M. Efficacy of a new dual channel laryngeal mask airway, the LMA® GastroTM Airway, for upper gastrointestinal endoscopy: a prospective observational study. Br J Anaesth. 2018; 120:353–360.
31. Goudra BG, Singh PM, Sinha AC. Anesthesia for ERCP: impact of anesthesiologist’s experience on outcome and cost. Anesthesiol Res Pract. 2013; 2013:570518.





 PDF
PDF Citation
Citation Print
Print








 XML Download
XML Download