INTRODUCTION
Even though the small intestine makes up 75% of the length of the digestive tract, small bowel malignancies are rare, accounting for less than 5% of gastrointestinal cancers. Annually, about 10,470 new patients are diagnosed with small bowel malignancies, while 1,450 patients die of them [
1-
4]. Owing to its nonspecific and ambiguous symptoms, small bowel malignancies are difficult to diagnose and may progress without symptoms [
5].
Various types of malignant tumors occur in the small bowel, including adenocarcinoma, gastrointestinal stromal tumors (GISTs), neuroendocrine tumors (NETs), and lymphoma [
2]. In the past, diagnosis of small bowel malignancies in the early stages was difficult due to limitations of tools. However, the introduction of double-balloon enteroscopy (DBE) has enabled the full evaluation of the entire small bowel, which dramatically improved the diagnosis and treatment of small bowel lesions, including obscure gastrointestinal bleeding and small bowel tumors [
5-
12]. Nowadays, video capsule endoscopy (VCE), DBE, and abdominal computed tomography (CT) are used to diagnose small bowel malignancy. Each tool has its advantages and disadvantages.
VCE is widely used to diagnose small bowel diseases because it is non-invasive and relatively easy to perform. VCE allows for visualization of the small bowel mucosa and its abnormalities on video [
7,
13,
14].
DBE not only enables full-length visual inspection of the small bowel, but also allows for direct intervention in the deep portions of the jejunum and ileum. It is therefore the most innovative diagnostic tool for small bowel diseases. DBE yields a high rate of successful diagnosis, but it has some limitations, including abdominal pain, side effects from anesthesia or endoscopy, and large economic burden [
5,
9,
10,
12,
15,
16].
There is no single best way to diagnose small bowel disease because the currently available tools complement each other. Therefore, the choice of tool for evaluation should be made according to individual patient complaints and conditions [
7,
12,
17].
In this study, we retrospectively analyzed the clinicopathological features of small bowel malignant tumors that were diagnosed with VCE, DBE, or abdominal CT in a single tertiary center.
Go to :

MATERIALS AND METHODS
Patients
Between January 2010 and September 2018, 438 patients underwent VCE or DBE at Guro Hospital, Korea University. We retrospectively analyzed the findings of VCE, DBE, and abdominal CT as well as their clinicopathologic characteristics. This study was approved by The Ethics Commission of Korea University, Guro Hospital.
Video capsule endoscopy
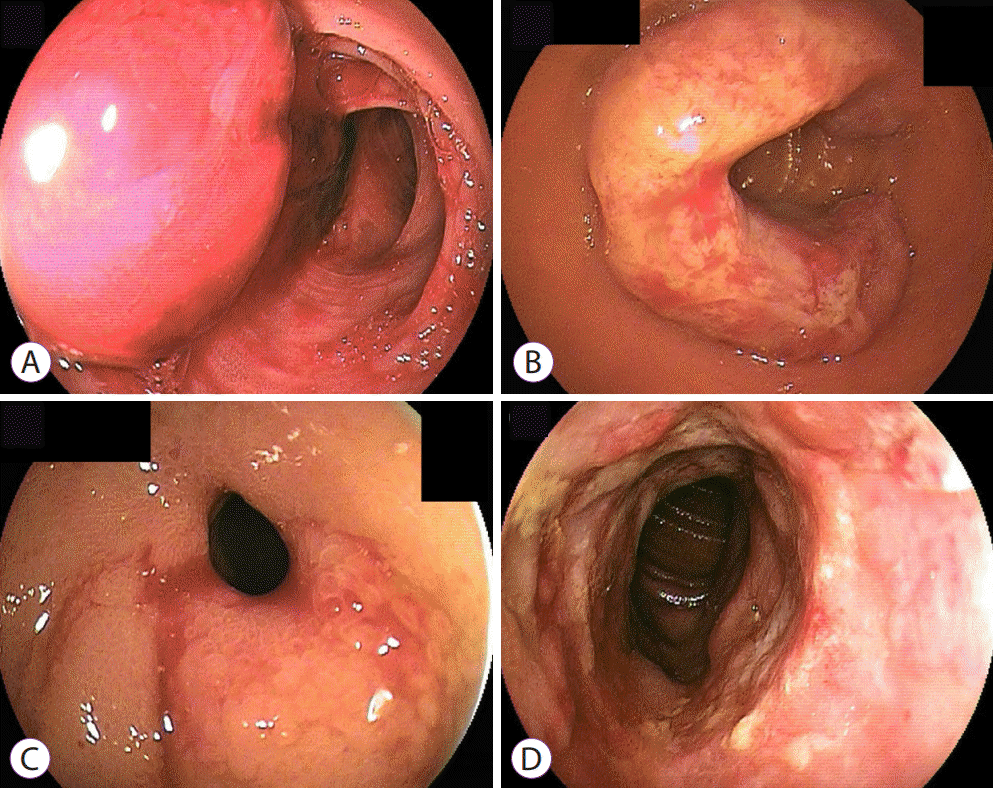
VCE was performed with MiroCam® (IntroMedic, Seoul, Korea). Laxatives were used for bowel preparation and the capsule was ingested 2 hours thereafter. The video was recorded for 12 hours, and the capsule was retrieved 24 hours after ingestion. The recorded videos were then analyzed by two enteroscopists, and the lesions were differentiated into three categories: submucosal, ulceroinfiltrative, and bleeding.
Double-balloon enteroscopy
DBE was performed using EN-450T5 (Fujinon Toshiba ES Systems, Tokyo, Japan). Patients were sedated with midazolam and propofol intravenously. DBE procedures were performed by a single endoscopist (BJL) and were predominantly indicated for tissue confirmation or therapeutic interventions including hemostasis, polypectomy, or foreign body removal. The insertion route was determined by the location of the lesion as evaluated by abdominal CT or VCE. During DBE, biopsy was performed on all lesions. The lesions were then differentiated into four categories, namely, ulceroinfiltrative, submucosal, ulcerofungating, and ulcers with stricture. Every patient underwent either VCE or DBE, and the choice for first-line diagnostic modality depended on the physician.
Abdominal computed tomography
Abdominal CT was performed either before or after VCE or DBE. The modality of choice may be non-enhanced, enhanced, or enteroclysis CT, based on the judgment of the physician. In some cases, CT was performed multiple times on one patient.
Analyses
Patients’ clinical features and their disease-specific pathologies were analyzed and compared for VCE, DBE and abdominal CT findings. SPSS 11.5 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Continuous variables are presented as mean±standard deviation. Categorical variables were analyzed using the chi-square test or Fisher’s exact test. A p-value lower than 0.05 was considered statistically significant.
Go to :

DISCUSSION
Small bowel malignancy is challenging to diagnose because the small bowel is difficult to evaluate. Diagnosis is further challenged owing to the nonspecific symptoms and, sometimes, the absence of symptoms during malignancy progression. Diagnosis and treatment are therefore usually delayed, and the consequences tend to be serious [
18-
20]. Abdominal CT is a valuable tool for diagnosing patients with small bowel malignancies, but there are many cases in which abdominal CT cannot provide useful information. Risk factor analyses may facilitate the diagnostic process, but the risk factors for small bowel cancer are difficult to predict because of its rarity and the various types of tumors that may be involved [
7].
After development of VCE in 2000, the entire small bowel and its lumen could be fully evaluated. VCE is relatively non-invasive, and is often used when small bowel malignancy is suspected following abdominal CT. However, VCE has its limitations, including false positives and false negatives as well as limited options for immediate action when malignancy is found [
9,
10].
Since the introduction of DBE in the early 2000s, a large amount of data has proven its diagnostic value for patients with small bowel pathologies. Like VCE, DBE allows full examination of the entire small bowel, which can help identify pathologies such as bleeding, strictures, and tumors. However, DBE can also be used for a variety of therapeutic interventions, including hemostasis, polypectomy, endoscopic mucosal resection, balloon dilatation, and stent insertion [
7,
12,
17].
Abdominal CT, VCE, and DBE are not meant to be used exclusively, but to complement each other depending on circumstances such as patient condition [
7,
12]. According to a guideline for enteroscopy published in Japan in 2017, VCE is recommended as a first-line diagnostic modality for obscure gastrointestinal bleeding, but is contraindicated for obstructive symptoms. It also suggests CT, magnetic resonance imaging, and enteroclysis as first diagnostic modalities for large tumors in the small intestine, and VCE for easier observation of small neoplasms or flat lesions. Thus, when evaluating the small bowel, it is necessary to perform the appropriate modalities depending on the different suspected conditions [
21].
Abdominal CT and VCE were used as screening examinations for small bowel diseases, while DBE was used for tissue biopsy and therapeutic interventions. For example, after achieving a diagnosis through abdominal CT and VCE, DBE would be performed if a biopsy or therapeutic intervention is needed. As DBE is more invasive than abdominal CT and VCE, it is unfeasible to be performed in every patient. Moreover, the requirement for sedation to reduce abdominal discomfort and pain may cause side effects such as desaturation and allergic reactions. There is also the possibility of endoscopic side effects, including bleeding and perforation. Finally, financial burden is another concern in patients undergoing endoscopy. For these reasons, DBE was only performed on selected patients who needed it the most [
7,
12,
17].
In our study, 28 patients were diagnosed with small bowel malignancy. The most common signs and symptoms were obscure bleeding (11/28, 39.3%), as well as abdominal pain and weight loss. The clinical signs varied depending on malignancy subtype. In cases of lymphoma, abdominal pain, including obstructive symptoms, was most common (4/8, 50%), whereas obscure bleeding was most common in cases of GIST (6/7, 85.7%). The presenting symptoms or clinical signs may be explained by the endoscopic features of each tumor type. For example, malignant lymphomas harbor encircling and ulcerative lesions, whereas a mass with ulceration is the major finding of GIST.
In a previous study, abnormal imaging findings were the most common clinicopathologic features of small bowel tumors, and the most common symptoms were bleeding and abdominal pain, including obstructive symptoms [
5]. In the present study, 11 subjects (39.3%) showed bleeding, and 8 (28.6%) showed abdominal pain and obstructive symptoms. Moreover, all 438 patients in this study underwent abdominal CT, and 27 of the 28 patients (96.4%) who were diagnosed with small bowel malignancy had positive CT findings, including heterogeneous wall thickening or masses (in all cases of GIST, adenocarcinoma, and metastatic cancer). However, in terms of lymphomas, 2 cases of MALT lymphoma showed positive endoscopic findings despite negative CT results. Abdominal CTs showed high sensitivity for diagnosing small bowel malignancy, but a positive finding itself cannot diagnose the exact subtype. As exemplified by the 2 cases of lymphoma with negative abdominal CT findings, additional examinations with DBE or VCE are required regardless of CT findings.
In the present study, tumor location significantly differed according to the type of malignancy. GIST was mainly located in the proximal small bowel, whereas malignant lymphoma was located at the distal portion of the duodenum. Horie et al. reported in 2019 that they diagnosed 16 epithelial tumors, 22 small bowel lymphomas, and 6 GISTs in 1,328 patients with DBEs [
22]. However, they failed to show statistical significance between tumor locations in the small bowel and the types of cancer. In this study, small bowel tumors were classified into 4 types; 71.4% of GIST was observed in the jejunum, 100% of lymphoma in the ileum, 62.5% of adenocarcinoma in the jejunum, and 80% of metastatic cancer in the jejunum. The study period was shorter and the number of subjects was smaller than those in Horie et al., but it showed statistically significant results with a
p-value of 0.002 [
22]. Therefore, abdominal CT findings in a specific anatomical location may be helpful in predicting the properties of small bowel malignancy and in planning for future treatment approaches.
There was no case of NET in our study, and this was in line with studies conducted in other Asian countries [
8,
15,
23]. In a Korean multicenter study, the most common small bowel tumors were GIST (
n=29, 25.9%), lymphoma (
n=18, 16.1%), and adenocarcinoma (
n=14, 12.5%) [
8]. In a Taiwanese study on 1,070 cases of DBE, the most common type was lymphoma (
n=20, 29.0%), followed by GIST (
n=19, 27.5%), adenocarcinoma (
n=18, 26.1%), metastatic cancer (
n=10, 14.5%), and only a few cases of NET (
n=2, 0.9%) [
15]. In contrast, NET was the most common type of small bowel malignancy in Western studies. Cangemi et al. showed that in 1,652 DBEs, the most common lesions were NET (19.4%), GIST (7.5%), and lymphoma (7.5%) [
5].
Regarding the prevalence of NET, a study from the United States showed that out of 25,531 cases of gastrointestinal NET, 38% occurred in the small bowel and 34% occurred in the rectum [
24]. In contrast, a Korean study showed that a majority of the cases of gastrointestinal NET were detected in the rectum (48%), 15% in the stomach, and 9% in the pancreas [
18]. The reasons for this epidemiological difference are unclear. Differences in ethnicity and the environment of clinical practice between Western and Asian countries might influence the location of gastrointestinal NET. Further research is therefore needed.
One limitation of this study is its retrospective-nature, which posed a possibility of bias. In addition, the small number of small bowel malignancy cases (28 patients) led to statistically nonsignificant results, although a trend could be drawn. However, we focused on the malignant cancer patients exclusively, and we had 438 patients who received small bowel evaluation, which is not few when compared to the other studies.
In conclusion, adenocarcinoma, malignant lymphoma, and GISTs were observed to be the most common small bowel malignant tumors. In our study, 28 of 438 patients who underwent VCE or DBE were diagnosed with small bowel malignancy, and tumor location significantly differed by tumor type. These findings elucidated the different clinical characteristics of each type of small bowel malignancy and hence merit further study.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download