Abstract
Objective
Methods
Results
Notes
Conceptualization: Yoon JA, Shin YI. Methodology: Yoon JA, Shin YI. Formal analysis: Yoon JA, Shin YI, Park MS. Funding acquisition: Kim YH. Project administration: all authors. Visualization: all authors. Writing – original draft: Yoon JA, Shin YI. Writing - review and editing: Yoon JA, Shin YI. Approval of final manuscript: all authors.
ACKNOWLEDGMENTS
REFERENCES
Fig. 1.
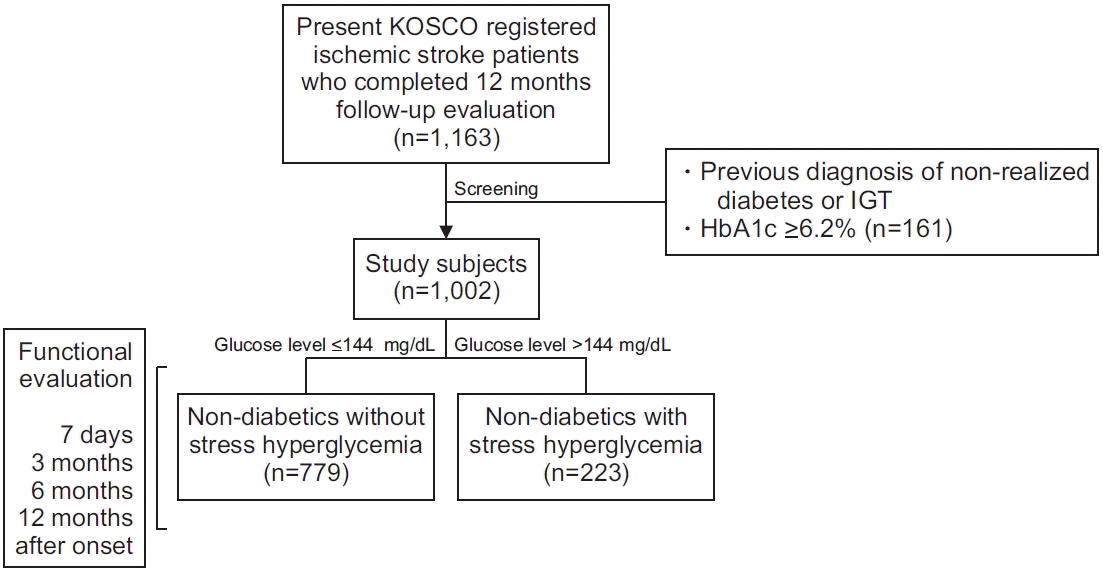
Fig. 2.
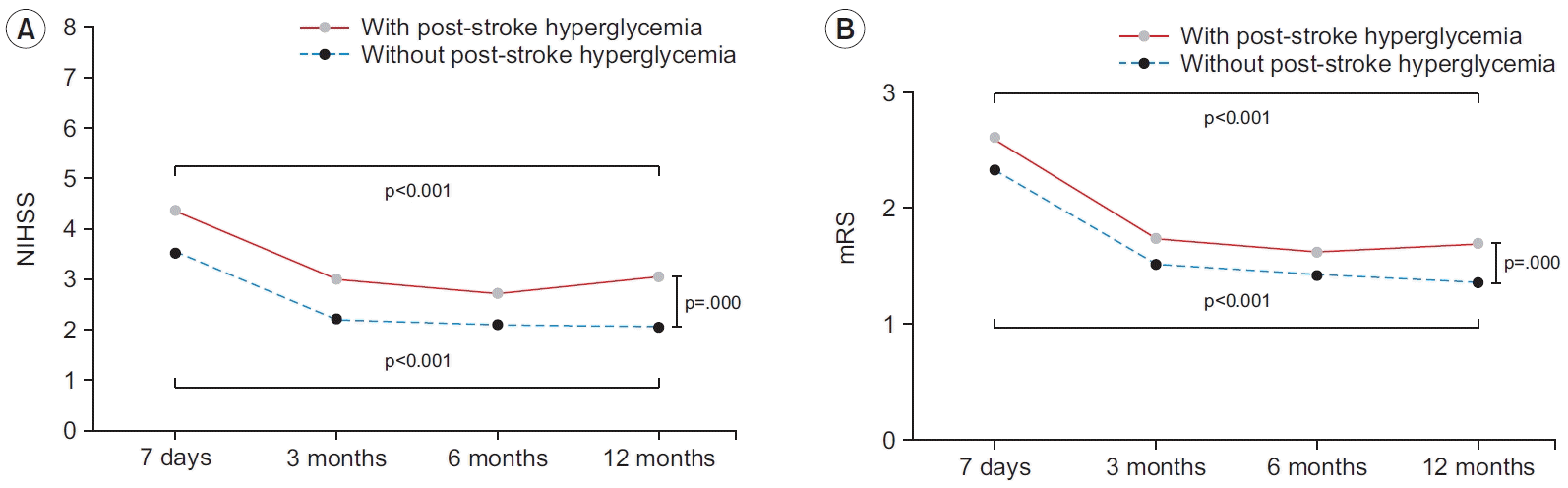
Table 1.
| Parameter | Total (n=1,002) | Non-diabetics without post-stroke hyperglycemia (n=779) | Non-diabetics with post-stroke hyperglycemia (n=223) | p-value | |
|---|---|---|---|---|---|
| Sex, male | 606 (60.5) | 481 (61.7) | 125 (56.1) | 0.125a) | |
| Age (yr) | 67.51±13.04 | 67.07±13.38 | 69.02±11.65 | 0.034c) | |
| BMI (kg/m2) | 23.42±3.00 | 23.38±2.94 | 23.55±3.24 | 0.471c) | |
| Waist circumference (cm) | 82.83±8.69 | 82.69±8.40 | 83.42±9.88 | 0.485c) | |
| Risk factors of stroke | |||||
| Hypertension | 504 (50.3) | 386 (49.6) | 118 (52.9) | 0.376a) | |
| Coronary heart disease | 36 (3.6) | 35 (4.5) | 4 (1.8) | 0.167a) | |
| Atrial fibrillation | 84 (8.4) | 82 (10.6) | 22 (9.9) | 0.457a) | |
| Hyperlipidemia | 129 (12.9) | 99 (12.7) | 30 (13.5) | 0.770a) | |
| Unruptured intracranial aneurysm | 5 (0.5) | 5 (0.6) | 0 (0.0) | 0.593b) | |
| Obesity | 97 (9.7) | 78 (10.0) | 19 (8.5) | 0.607a) | |
| Family history | 79 (7.9) | 66 (8.5) | 13 (5.8) | 0.197a) | |
| Smoking | 0.040a) | ||||
| Current smokers | 297 (29.6) | 244 (31.3) | 53 (23.8) | ||
| Former smokers | 113 (11.3) | 91 (11.7) | 22 (9.9) | ||
| Never smokers | 592 (59.1) | 444 (57.0) | 148 (66.4) | ||
| Alcohol consumption | 0.242a) | ||||
| None | 625 (62.4) | 496 (63.7) | 129 (57.8) | ||
| Moderate | 256 (25.5) | 190 (24.4) | 66 (29.6) | ||
| Heavy | 121 (12.1) | 93 (11.9) | 28 (12.6) | ||
| Ischemic type (TOAST) | 0.307a) | ||||
| Large-artery atherosclerosis | 472 (47.1) | 374 (48.0) | 98 (43.9) | ||
| Small-artery occlusion | 196 (19.6) | 158 (20.3) | 38 (17.0) | ||
| Cardioembolism | 149 (14.9) | 110 (14.1) | 39 (17.5) | ||
| Stroke of other determined etiology | 73 (7.3) | 56 (7.2) | 17 (7.6) | ||
| Stroke of undetermined etiology | 112 (11.2) | 81 (10.4) | 31 (13.9) | ||
| Ischemic location | 0.900c) | ||||
| Supratentorial lesion | 932 (93.0) | 725 (93.1) | 207 (92.8) | ||
| Infratentorial lesion | 70 (7.0) | 54 (6.9) | 16 (7.2) | ||
| Hospital arrival time after onset (hr) | 4.02±4.39 | 4.00±4.47 | 4.10±4.13 | 0.811c) | |
| Initial NIHSS score | 5.29±5.73 | 5.17±5.56 | 5.71±6.31 | 0.211c) | |
| Initial blood parameter | |||||
| Glucose (mg/dL) | 128.06±38.1 | 113.08±15.60 | 180.39±46.60 | 0.000c) | |
| Total cholesterol (mg/dL) | 177.48±51.3 | 176.54±52.00 | 180.74±48.70 | 0.282c) | |
Table 2.
|
Non-diabetics without post-stroke hyperglycemia (n=779) |
Non-diabetics with post-stroke hyperglycemia (n=223) |
p-value (inter-group) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 days | 3 months | 6 months | 12 months | p-value | 7 days | 3 months | 6 months | 12 months | p-value | ||
| NIHSS | 3.54±5.34 | 2.22±4.33 | 2.08±4.37 | 2.05±4.38 | 0.000* | 4.36±6.39 | 3.00±5.74 | 2.72±5.77 | 3.03±6.00 | 0.000* | 0.000* |
| FMA | 82.31±30.70 | 86.97±26.65 | 87.31±26.39 | 87.41±26.63 | 0.000* | 78.63±33.11 | 84.77±28.58 | 84.29±29.43 | 83.22±30.54 | 0.000* | 0.004* |
| FAC | 3.19±1.81 | 4.13±1.55 | 4.22±1.52 | 4.21±1.53 | 0.000* | 2.76±1.98 | 3.91±1.68 | 4.06±1.63 | 3.98±1.72 | 0.000* | 0.000* |
| mRS | 2.33±1.57 | 1.52±1.50 | 1.42±1.50 | 1.36±1.52 | 0.000* | 2.61±1.55 | 1.74±1.55 | 1.62±1.55 | 1.69±1.61 | 0.000* | 0.000* |
| K-MMSE | 21.73±9.20 | 23.50±8.46 | 23.74±8.41 | 23.72±8.73 | 0.000* | 20.50±10.21 | 22.91±8.90 | 23.57±8.20 | 23.04±8.81 | 0.000* | 0.051 |
| K-MBI | - | 86.63±26.17 | 87.24±26.72 | 87.72±26.59 | 0.057 | - | 82.86±29.24 | 84.07±29.16 | 83.84±30.35 | 0.183 | 0.004* |
| FIM | - | 109.40±28.28 | 110.23±28.51 | 110.54±28.86 | 0.045 | - | 105.09±31.49 | 106.76±31.18 | 106.44±32.13 | 0.563 | 0.007* |
Values are presented as mean±standard deviation.
NIHSS, National Institutes of Health Stroke Scale; FMA, Fugl-Meyer Assessment (affected side); FAC, Functional Ambulation Categories; mRS, modified Rankin Scale; K-MMSE, Korean Mini-Mental State Examination; K-MBI, Korean version of Modified Barthel Index; FIM, Functional Independence Measure.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download