Abstract
Objective
Methods
Results
Conclusion
Notes
Conceptualization: Cho WS, Chung SG, Kim W, Jo CH, Lee SH, Lee SY. Data curation: Cho WS, Lee SY. Formal analysis: Cho WS, Lee SY. Funding acquisition: Lee SY. Investigation: Cho WS, Chung SG, Kim W, Jo CH, Lee SH, Lee SY. Methodology: Cho WS, Lee SY. Project administration: Cho WS, Lee SY. Resources: Cho WS, Lee SY. Supervision: Chung SG, Kim W, Jo CH, Lee SH. Visualization: Cho WS, Lee SY. Writing – original draft: Cho WS, Lee SY. Writing – review & editing: Cho WS, Chung SG, Kim W, Jo CH, Lee SH, Lee SY.
ACKNOWLEDGMENTS
SUPPLEMENTARY MATERIALS
Supplement B.
REFERENCES
Fig. 1.
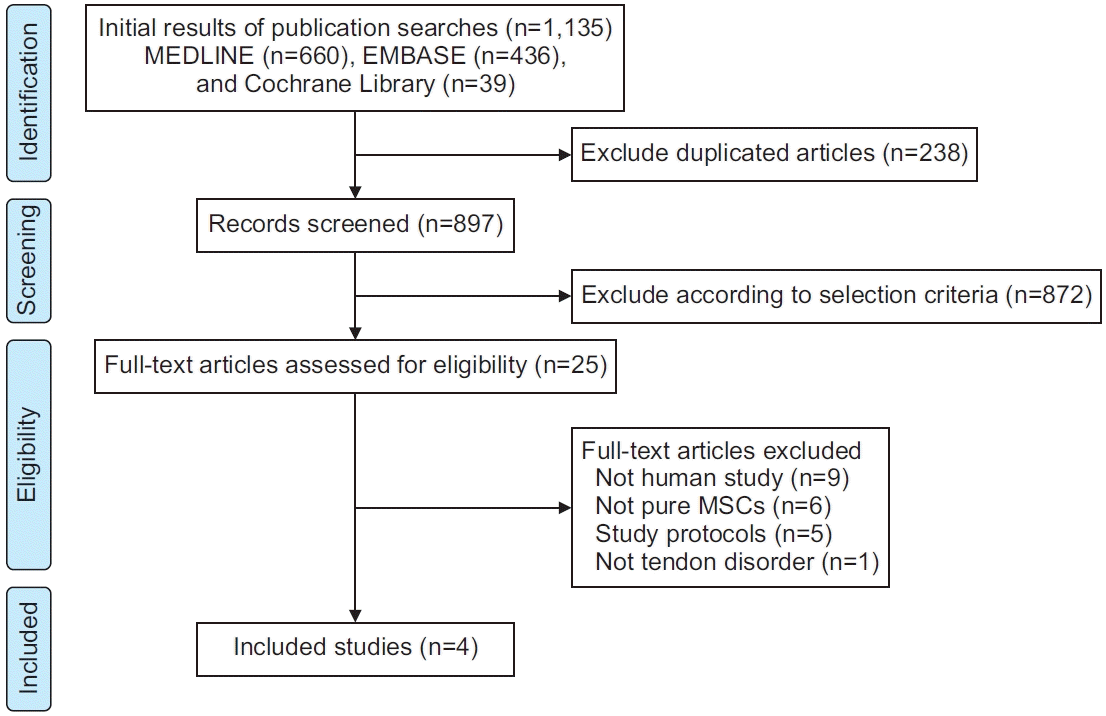
Fig. 2.
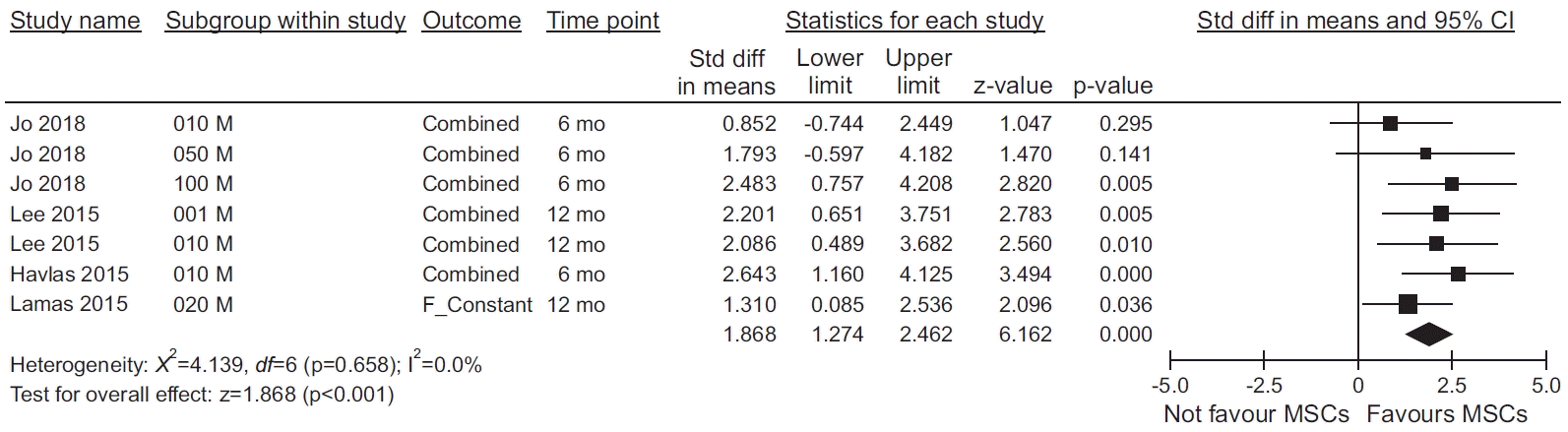
Fig. 3.
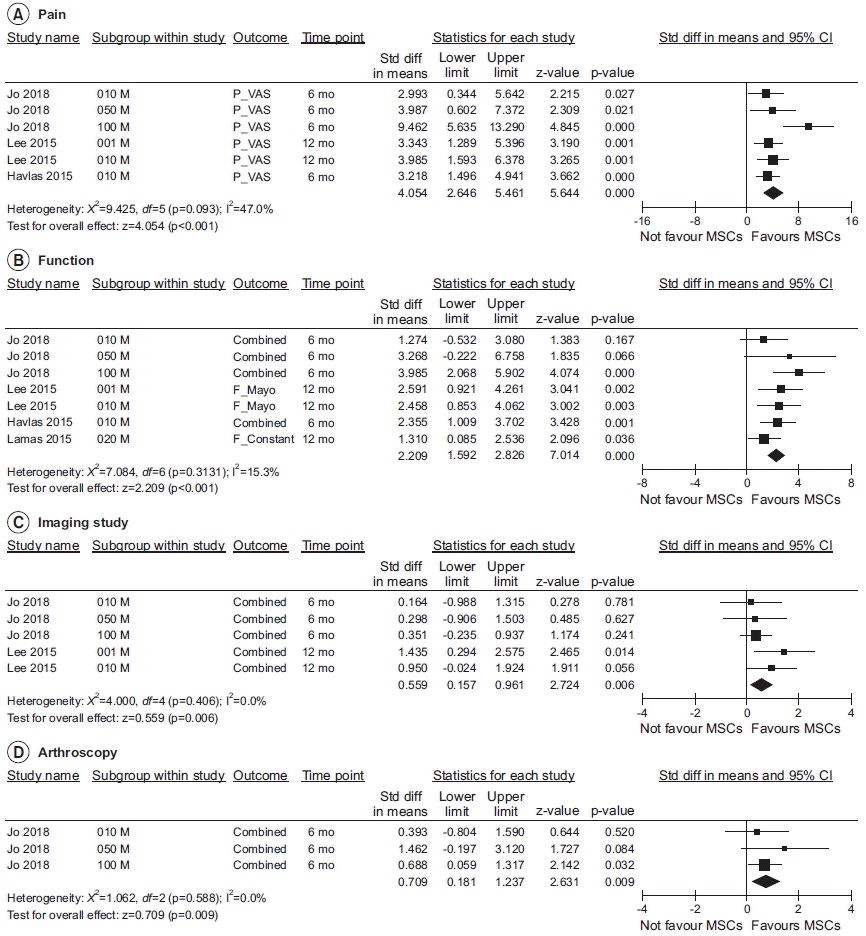
Fig. 4.
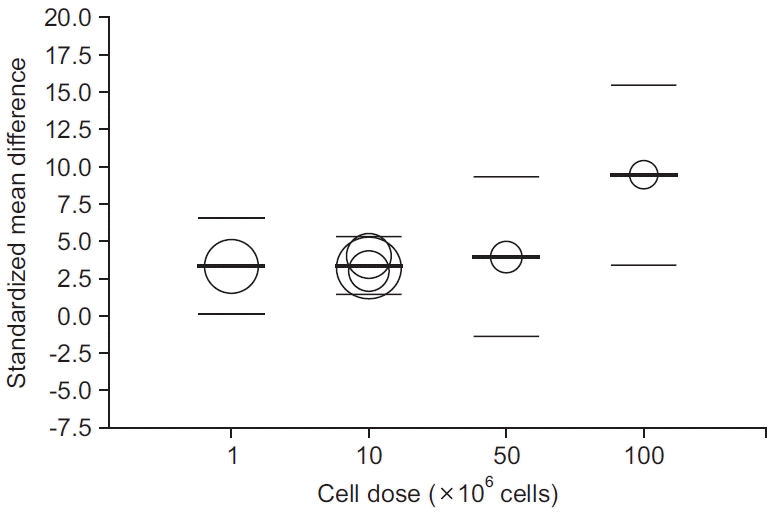
Table 1.
| Study | Region | Study period | Study design | N | Tendon disorder | MSC origin (type) | Injection method | MSC dose (cells) | F/U period (mo) | Safety |
Efficacy |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary | Secondary | |||||||||||
| Lamas et al. [11], 2019 | Spain | Jan 2011–Nov 2012 | Double-blind randomized controlled trial | 13 | Full-thickness rotator cuff tear | Autologous bone marrow-derived MSCs | Surgical repair with attached with OrthADAPT membrane | 2.0×107 | 12 | Examined but not mentioned (the trial was stopped due to adverse effects) | Constant score | Tendon status by MRI pain (VAS) |
| Jo et al. [19], 2018 | South Korea | Jul 2015–Nov 2016 | Open-label, dose-escalation trial | 19 | Partial-thickness rotator cuff tear | Autologous adipose tissue-derived MSCs | Intratendinous injection under the US guidance, MSCs in 3 mL of saline | 1.0×107, 5.0×107, 1.0×108 | 6 | NCI-CTCAE v 4.0 | SPADI | Constant score, pain (VAS) shoulder MRI (tendon defects), arthroscopy |
| Lee et al. [8], 2015 | South Korea | May 2013–Sep 2014 | Open-label, conventional 3+3 cohort expansion design | 12 | Lateral epicondylitis | Allogeneic adipose tissue-derived MSCs | Intratendinous injection under the US guidance, MSCs with fibrin glue (total volume of 1 mL) | 1.0×106, 1.0×107 | 12 | Local/systemic tolerances, US exam | Pain (VAS) | Modified mayo elbow performance index, elbow US (tendon defects) |
| Havlas et al. [10], 2015 | Czech Republic | Oct 2012 | Prospective study with consecutive participants | 8 | Rotator cuff tear | Autologous bone marrow-derived MSCs | Arthroscopic repair and suspension of MSCs to the suture site | (1.0±0.45)×107 | 6 | Local and systematic adverse reactions (not clearly described) | Pain (VAS) | Constant score, UCLA score |
MSCs, mesenchymal stem cells; F/U, follow-up; MRI, magnetic resonance imaging; VAS, visual analog scale; US, ultrasonography; NCI-CTCAE, National Cancer Institute-Common Terminology Criteria for Adverse Events; SPADI, Shoulder Pain and Disability Index; UCLA, University of California at Los Angeles.
Table 2.
| Study | Adverse events | N | Treatment | Prognosis | Treatment-related |
|---|---|---|---|---|---|
| Lamas et al. [11], 2019 | Supraclavicular cyst and subacromial inflammatory tissue (foreign body like reaction) | 4 | Surgery (remove the scaffold) | Recovered | Yes |
| Jo et al. [19], 2018 | Back pain | 3 | Rescue drug, physical therapy | Recovered | No |
| Right foot bruise, left trigger finger | 1 | Rescue drug, physical therapy | Recovered | No | |
| Cough | 1 | Medication | Recovered | No | |
| Left eye pain | 1 | Eye drop | Recovered | No | |
| Abdominal pain | 1 | Medication | Recovered | No | |
| Lee et al. [8], 2015 | Mild regional swelling | 6 | Observation | Recovered | Yes |
| Mild elbow joint effusion | 2 | Observation | Recovered | Yes | |
| Delayed elbow pain | 1 | Rescue drug | Recovered | No |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download