1. Gupta R, Mohan I, Narula J. Trends in coronary heart disease epidemiology in India. Ann Glob Health. 2016; 82:307–15.

2. GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017; 390:1151–210.
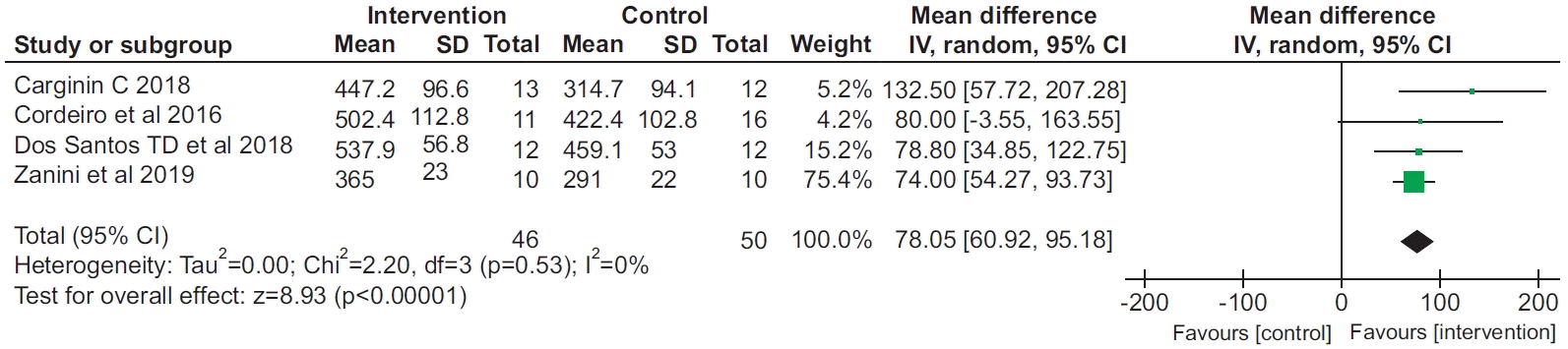
3. Dos Santos TD, Pereira SN, Portela LO, Cardoso DM, Lago PD, Dos Santos Guarda N, et al. Moderate-tohigh intensity inspiratory muscle training improves the effects of combined training on exercise capacity in patients after coronary artery bypass graft surgery: a randomized clinical trial. Int J Cardiol. 2019; 279:40–6.

4. Sibilitz KL, Berg SK, Hansen TB, Risom SS, Rasmussen TB, Hassager C, et al. Effect of comprehensive cardiac rehabilitation after heart valve surgery (CopenHeart VR): study protocol for a randomised clinical trial. Trials. 2013; 14:1–14.
5. Haeffener MP, Ferreira GM, Barreto SS, Arena R, Dall’Ago P. Incentive spirometry with expiratory positive airway pressure reduces pulmonary complications, improves pulmonary function and 6-minute walk distance in patients undergoing coronary artery bypass graft surgery. Am Heart J. 2008; 156:900.e1–900.e8.

6. Wynne R, Botti M. Postoperative pulmonary dysfunction in adults after cardiac surgery with cardiopulmonary bypass: clinical significance and implications for practice. Am J Crit Care. 2004; 13:384–93.

7. Shakouri SK, Salekzamani Y, Taghizadieh A, SabbaghJadid H, Soleymani J, Sahebi L, et al. Effect of respiratory rehabilitation before open cardiac surgery on respiratory function: a randomized clinical trial. J Cardiovasc Thorac Res. 2015; 7:13–7.

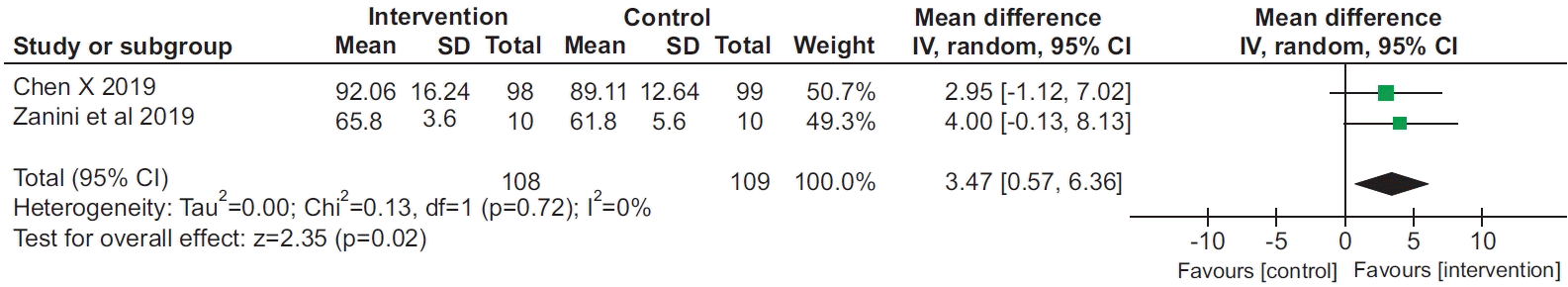
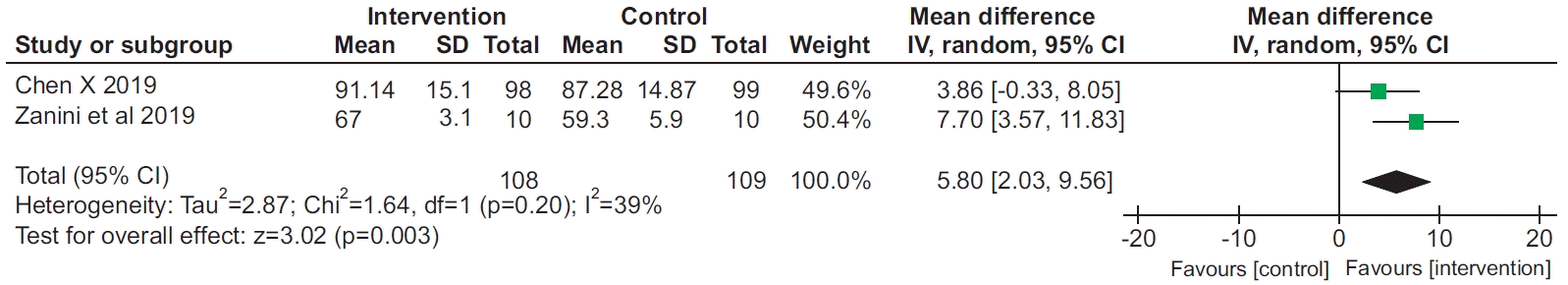
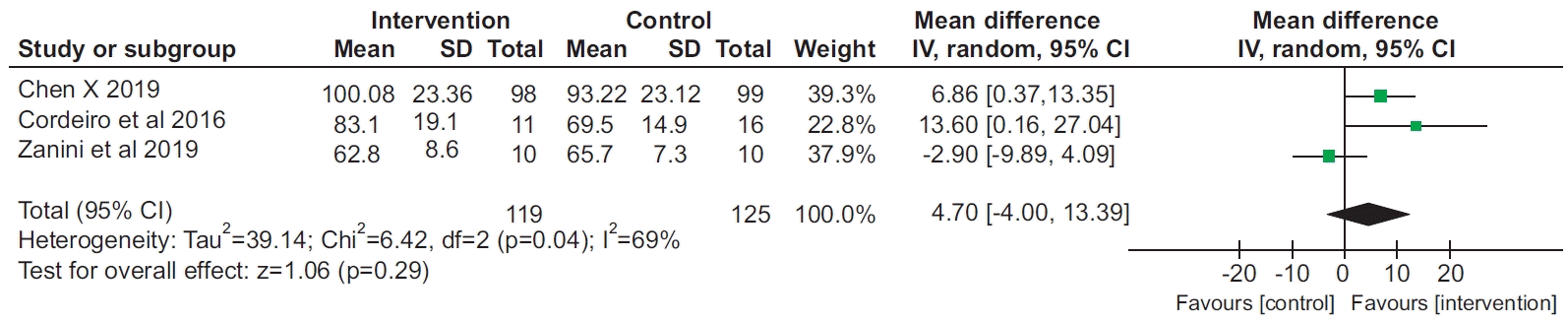
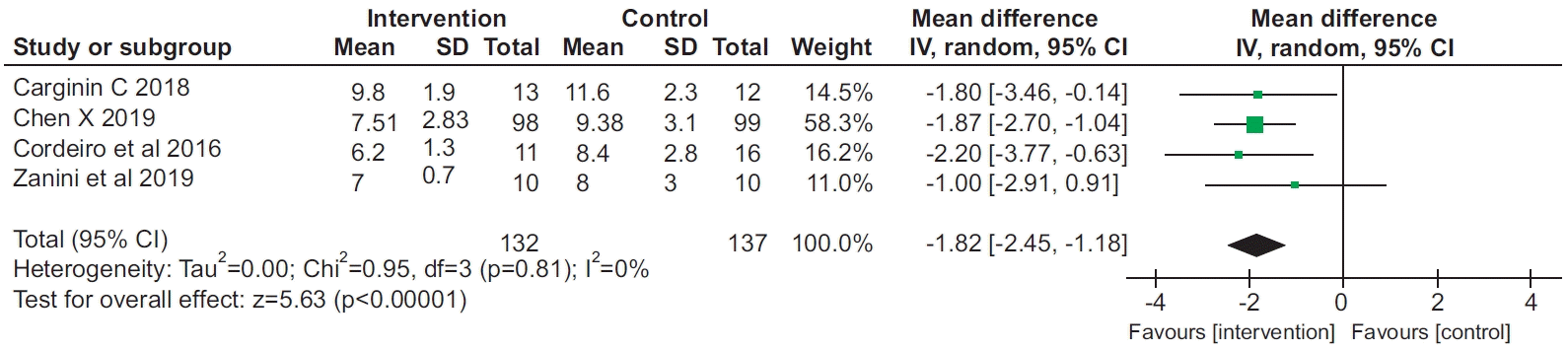
8. Chen X, Hou L, Zhang Y, Liu X, Shao B, Yuan B, et al. The effects of five days of intensive preoperative inspiratory muscle training on postoperative complications and outcome in patients having cardiac surgery: a randomized controlled trial. Clin Rehabil. 2019; 33:913–22.

9. Baumgarten MC, Garcia GK, Frantzeski MH, Giacomazzi CM, Lagni VB, Dias AS, et al. Pain and pulmonary function in patients submitted to heart surgery via sternotomy. Rev Bras Cir Cardiovasc. 2009; 24:497–505.
10. Praveen R, Swaminathan N, Praveen JS. Inspiratory muscle training is effective in improving respiratory muscle functions in patients who have undergone coronary artery bypass graft. Fizjoterapia Polska. 2009; 9:285–92.
11. Siafakas NM, Mitrouska I, Bouros D, Georgopoulos D. Surgery and the respiratory muscles. Thorax. 1999; 54:458–65.

12. Westerdahl E, Jonsson M, Emtner M. Pulmonary function and health-related quality of life 1-year follow up after cardiac surgery. J Cardiothorac Surg. 2016; 11:99.

13. Westerdahl E, Lindmark B, Bryngelsson I, Tenling A. Pulmonary function 4 months after coronary artery bypass graft surgery. Respir Med. 2003; 97:317–22.

14. Mans CM, Reeve JC, Elkins MR. Postoperative outcomes following preoperative inspiratory muscle training in patients undergoing cardiothoracic or upper abdominal surgery: a systematic review and meta analysis. Clin Rehabil. 2015; 29:426–38.

15. Savci S, Degirmenci B, Saglam M, Arikan H, Inal-Ince D, Turan HN, et al. Short-term effects of inspiratory muscle training in coronary artery bypass graft surgery: a randomized controlled trial. Scand Cardiovasc J. 2011; 45:286–93.

16. Cordeiro AL, de Melo TA, Neves D, Luna J, Esquivel MS, Guimaraes AR, et al. Inspiratory muscle training and functional capacity in patients undergoing cardiac surgery. Braz J Cardiovasc Surg. 2016; 31:140–4.
17. Cargnin C, Karsten M, Guaragna JC, Dal Lago P. Inspiratory muscle training after heart valve replacement surgery improves inspiratory muscle strength, lung function, and functional capacity: a randomized controlled trial. J Cardiopulm Rehabil Prev. 2019; 39:E1–E7.
18. Zanini M, Nery RM, de Lima JB, Buhler RP, da Silveira AD, Stein R. Effects of different rehabilitation protocols in inpatient cardiac rehabilitation after coronary artery bypass graft surgery: a randomized clinical trial. J Cardiopulm Rehabil Prev. 2019; 39:E19–E25.
19. McMahon SR, Ades PA, Thompson PD. The role of cardiac rehabilitation in patients with heart disease. Trends Cardiovasc Med. 2017; 27:420–5.

20. De Menezes TC, Bassi D, Cavalcanti RC, Barros JE, Granja KS, Calles AC, et al. Comparisons and correlations of pain intensity and respiratory and peripheral muscle strength in the pre- and postoperative periods of cardiac surgery. Rev Bras Ter Intensiva. 2018; 30:479–86.
21. Shenkman Z, Shir Y, Weiss YG, Bleiberg B, Gross D. The effects of cardiac surgery on early and late pulmonary functions. Acta Anaesthesiol Scand. 1997; 41:1193–9.

22. Hulzebos EH, Smit Y, Helders PP, van Meeteren NL. Preoperative physical therapy for elective cardiac surgery patients. Cochrane Database Syst Rev. 2012; 11:CD010118.

23. Gomes Neto M, Martinez BP, Reis HF, Carvalho VO. Pre- and postoperative inspiratory muscle training in patients undergoing cardiac surgery: systematic review and meta-analysis. Clin Rehabil. 2017; 31:454–64.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download