INTRODUCTION
MATERIALS AND METHODS
Study design
Subjects
Methods
Statistical analyses
RESULTS
Baseline characteristics of the subjects
Table 1.
| Characteristic | CBCR (n=37) | HBCR (n=41) | p-value |
|---|---|---|---|
| Age (yr) | 63.5±9.3 | 62.1±9.6 | 0.53 |
| Sex | 0.29 | ||
| Male | 28 | 35 | |
| Female | 9 | 6 | |
| BMI (kg/m2) | 24.8±3.6 | 26.2±3.2 | 0.09 |
| LVEF (%) | 58.5±12.7 | 57.9±10.4 | 0.83 |
| Exercise capacity | |||
| VO2max (mL/kg/min) | 26.4±6.4 | 30.4±7.8 | 0.02* |
| METsmax | 7.6±1.8 | 8.9±2.6 | 0.01* |
| VO2AT | 15.3±4.5 | 15.7±3.4 | 0.73 |
| Smoking history | |||
| Never | 22 (59.5) | 13 (31.7) | 0.01* |
| Ex-smoker | 6 (16.2) | 10 (24.4) | 0.37 |
| Current | 9 (24.3) | 18 (43.9) | 0.07 |
| Cardiac diagnosis | |||
| STEMI | 16 (43.3) | 14 (34.2) | 0.41 |
| Non-STEMI | 7 (18.9) | 12 (29.3) | 0.29 |
| Unstable angina | 14 (37.8) | 15 (36.5) | 0.91 |
| Comorbidity | |||
| Hypertension | 21 (56.8) | 21 (56.8) | 0.48 |
| Diabetes mellitus | |||
| None | 28 (75.7) | 30 (73.2) | 0.80 |
| Non-IDDM | 8 (21.6) | 10 (24.4) | 0.76 |
| IDDM | 1 (2.7) | 1 (2.4) | 0.35 |
| Dyslipidemia | 13 (35.1) | 12 (29.3) | 0.58 |
| Heart failure | 4 (10.8) | 2 (4.9) | 0.59 |
| Others | 5 (13.5) | 8 (19.5) | 0.66 |
| None | 7 (18.9) | 5 (12.1) | 0.95 |
| Revascularization | |||
| PCI | 35 (94.6) | 39 (95.1) | 0.56 |
| CABG | 2 (5.4) | 2 (4.9) | 0.91 |
| Number of diseased vessels | 1.57±0.7 | 1.63±0.8 | 0.71 |
| Number of inserted stents | 1.24±0.7 | 1.12±0.6 | 0.39 |
| Number of follow-up CPX test | 9.1±1.8 | 10.0±2.5 | 0.72 |
Values are presented as mean±standard deviation or number (%).
CBCR, center-based cardiac rehabilitation; HBCR, home-based cardiac rehabilitation; BMI, body mass index; LVEF, left ventricle ejection fraction; VO2max, maximal oxygen consumption; METsmax, maximal metabolic equivalents; VO2AT, oxygen consumption at anaerobic threshold; STEMI, ST-segment elevation myocardial infarction; IDDM, insulin-dependent DM; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; CPX, cardiopulmonary exercise.
Table 2.
| CPX results |
CBCR (n=37) |
HBCR (n=41) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 wk | 6 mo | 1 yr | 3 yr | 5 yr | Baseline | 12 wk | 6 mo | 1 yr | 3 yr | 5 yr | |
| VO2max (mL/kg/min) | 26.4±6.4a) | 30.2±7.6b) | 30.9±8.8b) | 32.0±11.0b) | 28.5±8.0 | 26.4±6.5 | 30.4±7.8a) | 31.4±7.1 | 33.2±7.4b) | 33.1±8.3b) | 29.8±7.2 | 29.0±7.0 |
| METsmax (mL/kg/min) | 7.6±1.8a) | 8.6±2.2b) | 8.8±2.5b) | 9.2±3.1b) | 8.1±2.3 | 7.6±1.9 | 8.9±2.6a) | 9.0±2.0 | 9.5±2.1b) | 9.4±2.4 | 8.5±2.1 | 8.3±2.0 |
| VO2AT (mL/kg/min) | 15.3±4.5 | 17.1±5.0 | 18.2±4.6b) | 17.6±6.8 | 16.5±5.8 | 17.9±4.4b) | 15.7±3.4 | 18.0±4.5 | 17.1±6.5 | 17.9±5.8 | 18.5±5.0 | 19.9±5.1b) |
| RPP3/100 (mmHg∙bpm) | 148±38 | 131±28b) | 131±26b) | 135±33b) | 145±31 | 148±39 | 146±40 | 133±31b) | 136±42b) | 140±27 | 149±39 | 142±32 |
| RPE3 | 10.3±2.4 | 8.7±1.7b) | 9.4±1.7a) | 9.5±1.7a) | 10.0±2.0 | 9.8±1.7 | 9.7±2.8 | 8.3±1.6b) | 8.4±1.9a,b) | 8.6±1.6a,b) | 10.1±2.4 | 12.5±2.0 |
Values are presented as mean±standard deviation or number (%).
CPX, cardiopulmonary exercise; CBCR, center-based cardiac rehabilitation; HBCR, home-based cardiac rehabilitation; VO2max, maximal oxygen consumption; METsmax, maximal metabolic equivalents; VO2AT, oxygen consumption at anaerobic threshold; RPP3, rate pressure product at stage 3; RPE3, rate of perceived exertion at stage 3.
Comparison of CRF over time between the CBCR and HBCR groups
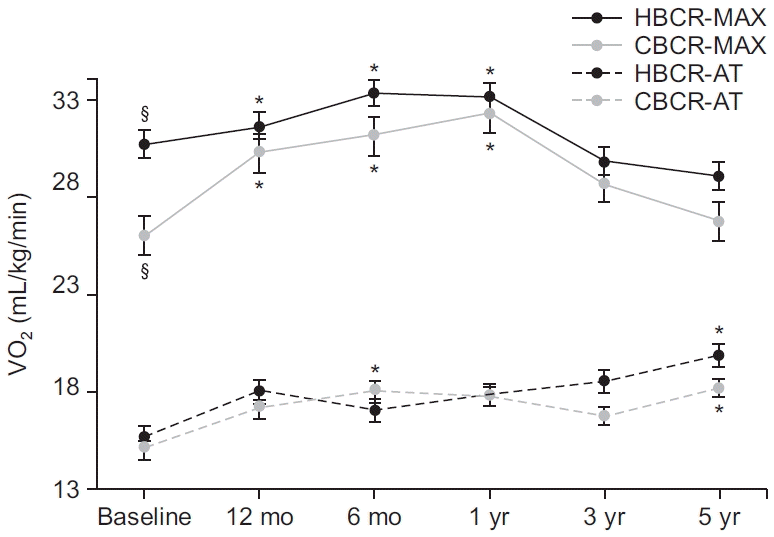 | Fig. 2.Five-year trends of VO2max and VO2AT in the CBCR and HBCR groups. CBCR, center-based cardiac rehabilitation; HBCR, home-based cardiac rehabilitation; MAX, maximal oxygen consumption; AT, oxygen consumption at anaerobic threshold. §p<0.05, significant difference between the two groups at the same time. *p<0.05, significantly different from the baseline value. |
Comparison of follow-up MET values over time between the baseline CRF levels of the CBCR and HBCR groups
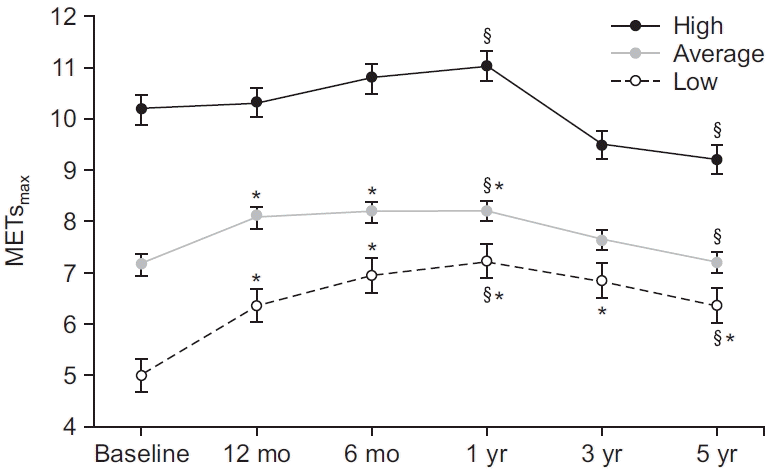 | Fig. 3.Five-year trends of maximal metabolic equivalents (METsmax) in three cardiorespiratory fitness groups: high (≥8 METs), average, (≥6 METs and <8 METs), and low (<6 METs). §p<0.05, significant difference between two periods in the same group. *p<0.05, significantly different from the baseline value. |
Table 3.
| CRF | Baseline | 12 wk | 6 mo | 1 yr | 3 yr | 5 yr |
|---|---|---|---|---|---|---|
| High | 10.2±1.9 | 10.2±1.7 | 10.8±1.9 | 11.0±2.6a) | 9.5±2.1 | 9.2±1.6a,b) |
| Average | 7.1±0.6 | 8.0±1.5b) | 8.2±1.4b) | 8.2±1.8a,b) | 7.6±1.6 | 7.2±1.6a) |
| Low | 5.0±0.6 | 6.7±1.5b) | 6.9±1.8b) | 7.2±2.2a,b) | 6.8±1.6b) | 6.3±1.4a,b) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download