2. Kweon S, Sohn MK, Jeong JO, Kim S, Jeon H, Lee H, et al. Quality of life and awareness of cardiac rehabilitation program in people with cardiovascular diseases. Ann Rehabil Med. 2017; 41:248–56.

3. WHO Expert Committee on Rehabilitation of Patients with Cardiovascular Diseases & World Health Organization. Rehabilitation of patients with cardiovascular diseases: report of a WHO Expert Committee (meeting held in Geneva from 23 to 29 July 1963). Geneva, Switzerland: World Health Organization;1964.
4. Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, et al. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol. 2016; 67:1–12.
5. Rauch B, Davos CH, Doherty P, Saure D, Metzendorf MI, Salzwedel A, et al. The prognostic effect of cardiac rehabilitation in the era of acute revascularisation and statin therapy: a systematic review and meta-analysis of randomized and non-randomized studies. The Cardiac Rehabilitation Outcome Study (CROS). Eur J Prev Cardiol. 2016; 23:1914–39.
6. Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn S, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013; 66:408–14.

7. Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011; 64:401–6.

8. West RR, Jones DA, Henderson AH. Rehabilitation after myocardial infarction trial (RAMIT): multi-centre randomised controlled trial of comprehensive cardiac rehabilitation in patients following acute myocardial infarction. Heart. 2012; 98:637–44.

9. Maroto Montero JM, Artigao Ramirez R, Morales Duran MD, de Pablo Zarzosa C, Abraira V. Cardiac rehabilitation in patients with myocardial infarction: a 10-year follow-up study. Rev Esp Cardiol. 2005; 58:1181–7.

10. Pouche M, Ruidavets JB, Ferrieres J, Iliou MC, Douard H, Lorgis L, et al. Cardiac rehabilitation and 5-year mortality after acute coronary syndromes: the 2005 French FAST-MI study. Arch Cardiovasc Dis. 2016; 109:178–87.

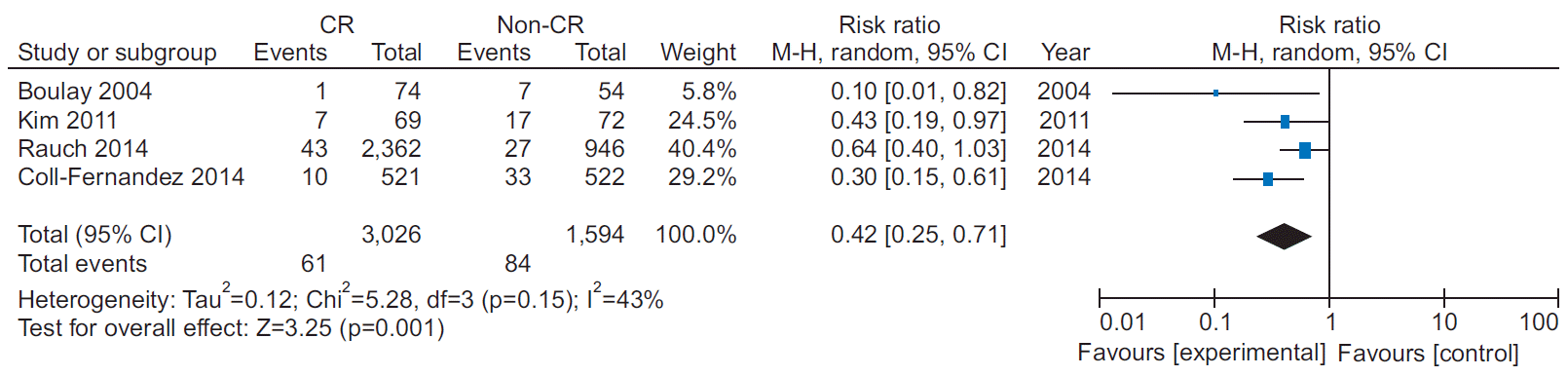
11. Coll-Fernandez R, Coll R, Pascual T, Sanchez MunozTorrero JF, Sahuquillo JC, Manzano L, et al. Cardiac rehabilitation and outcome in stable outpatients with recent myocardial infarction. Arch Phys Med Rehabil. 2014; 95:322–9.
12. Kureshi F, Kennedy KF, Jones PG, Thomas RJ, Arnold SV, Sharma P, et al. Association between cardiac rehabilitation participation and health status outcomes after acute myocardial infarction. JAMA Cardiol. 2016; 1:980–8.

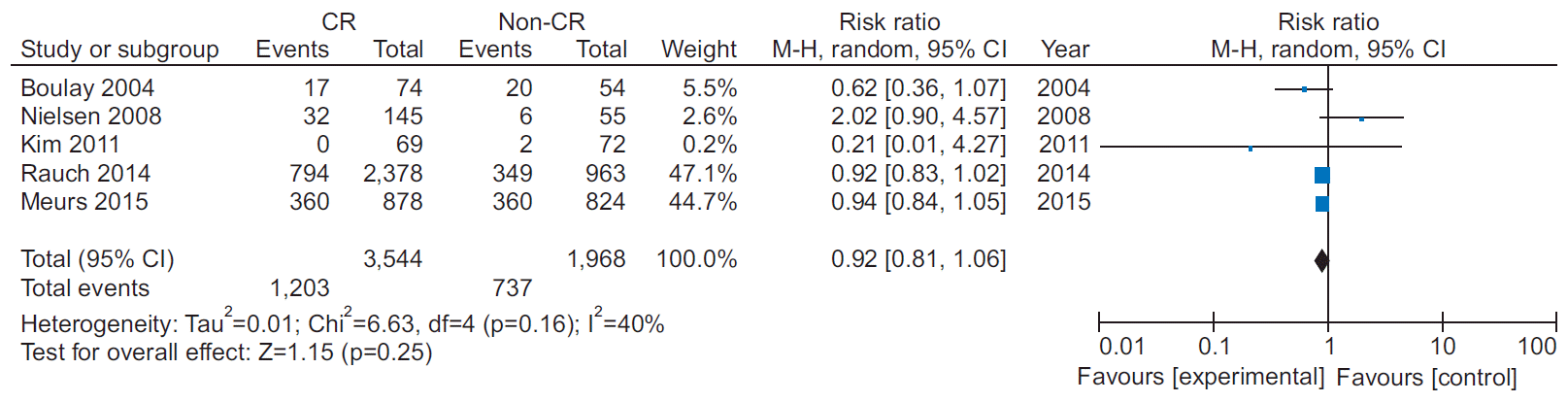
13. Kim C, Kim DY, Moon CJ. Prognostic influences of cardiac rehabilitation in Korean acute myocardial infarction patients. Ann Rehabil Med. 2011; 35:375–80.

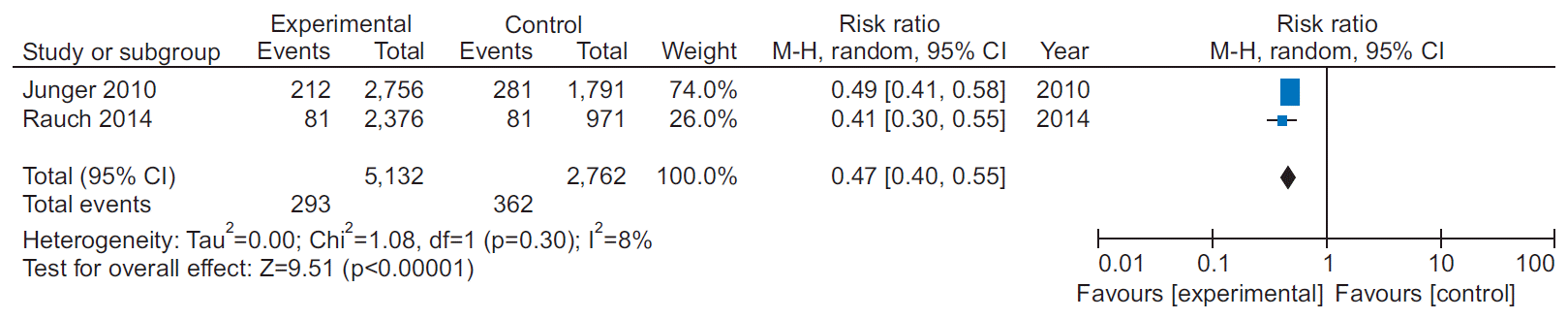
14. Junger C, Rauch B, Schneider S, Liebhart N, Rauch G, Senges J, et al. Effect of early short-term cardiac rehabilitation after acute ST-elevation and non-ST-elevation myocardial infarction on 1-year mortality. Curr Med Res Opin. 2010; 26:803–11.
15. Rauch B, Riemer T, Schwaab B, Schneider S, Diller F, Gohlke H, et al. Short-term comprehensive cardiac rehabilitation after AMI is associated with reduced 1-year mortality: results from the OMEGA study. Eur J Prev Cardiol. 2014; 21:1060–9.

16. Lewinter C, Bland JM, Crouch S, Doherty P, Lewin RJ, Kober L, et al. The effect of referral for cardiac rehabilitation on survival following acute myocardial infarction: a comparison survival in two cohorts collected in 1995 and 2003. Eur J Prev Cardiol. 2014; 21:163–71.

17. Meurs M, Burger H, van Riezen J, Slaets JP, Rosmalen JG, van Melle JP, et al. The association between cardiac rehabilitation and mortality risk for myocardial infarction patients with and without depressive symptoms. J Affect Disord. 2015; 188:278–83.

18. Beauchamp A, Worcester M, Ng A, Murphy B, Tatoulis J, Grigg L, et al. Attendance at cardiac rehabilitation is associated with lower all-cause mortality after 14 years of follow-up. Heart. 2013; 99:620–5.
19. Suaya JA, Stason WB, Ades PA, Normand SL, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol. 2009; 54:25–33.

20. Nielsen KM, Faergeman O, Foldspang A, Larsen ML. Cardiac rehabilitation: health characteristics and socio-economic status among those who do not attend. Eur J Public Health. 2008; 18:479–83.

21. Boulay P, Prud’homme D. Health-care consumption and recurrent myocardial infarction after 1 year of conventional treatment versus short- and long-term cardiac rehabilitation. Prev Med. 2004; 38:586–93.
22. Santiago de Araujo Pio C, Marzolini S, Pakosh M, Grace SL. Effect of cardiac rehabilitation dose on mortality and morbidity: a systematic review and meta-regression analysis. Mayo Clin Proc. 2017; 92:1644–59.
23. Mann DL, Zipes DP, Libby P, Bonow RO. Braunwald’s heart disease : a textbook of cardiovascular medicine. 10th ed. Philadelphia, PA: Elsevier/Saunders;2015.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download