1. Lilly SL. Pathophysiology of heart disease: a collaborative project of medical students and faculty. 5th Ed. Philadelphia, PA: Lippincott Williams & Wilkins;2011.
2. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016; 37:67–119.
3. D’Alto M, Merola A, Dimopoulos K. Pulmonary hypertension related to congenital heart disease: a comprehensive review. Glob Cardiol Sci Pract. 2015; 2015:42.
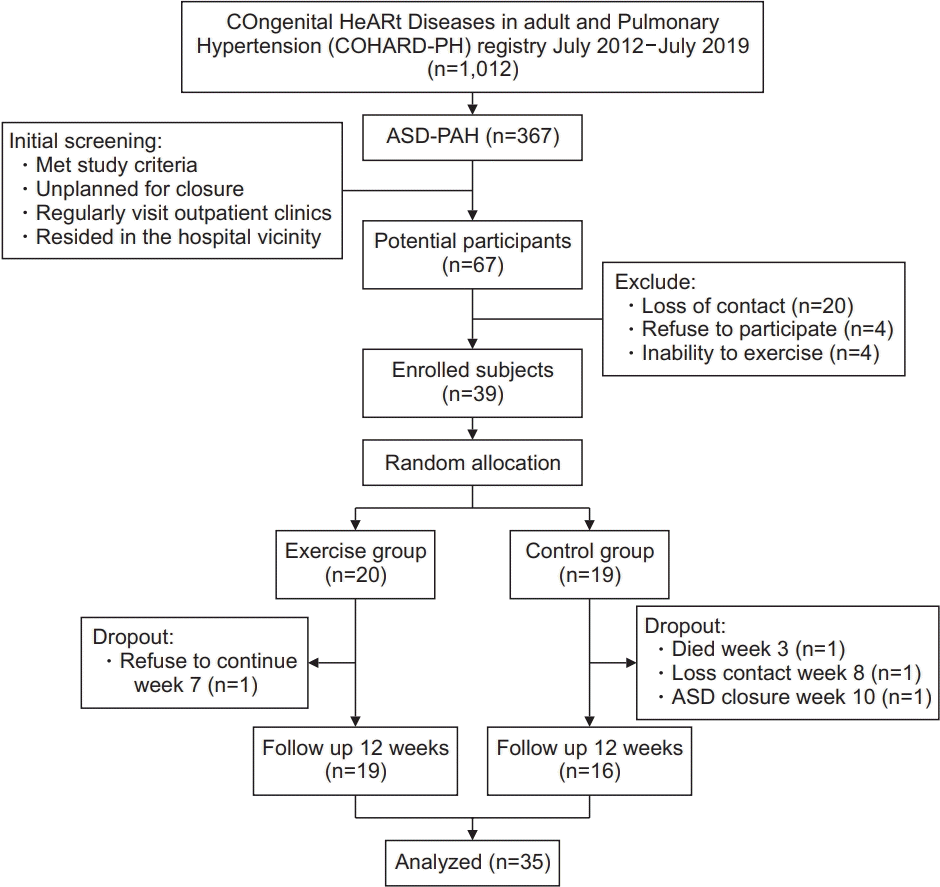
4. Dinarti LK, Hartopo AB, Kusuma AD, Satwiko MG, Hadwiono MR, Pradana AD, et al. The COngenital HeARt Disease in adult and Pulmonary Hypertension (COHARD-PH) registry: a descriptive study from single-center hospital registry of adult congenital heart disease and pulmonary hypertension in Indonesia. BMC Cardiovasc Disord. 2020; 20:163.

5. Vijarnsorn C, Durongpisitkul K, Chungsomprasong P, Bositthipichet D, Ketsara S, Titaram Y, et al. Contemporary survival of patients with pulmonary arterial hypertension and congenital systemic to pulmonary shunts. PLoS One. 2018; 13:e0195092.

6. Sahni S, Capozzi B, Iftikhar A, Sgouras V, Ojrzanowski M, Talwar A. Pulmonary rehabilitation and exercise in pulmonary arterial hypertension: an underutilized intervention. J Exerc Rehabil. 2015; 11:74–9.

7. Lilyasari O, Subekti Y, Atika N, Dinarti LK, Putri S, Opitasari C, et al. Economic evaluation of sildenafil for the treatment of pulmonary arterial hypertension in Indonesia. BMC Health Serv Res. 2019; 19:573.

8. Dalla Vecchia LAD, Bussotti M. Exercise training in pulmonary arterial hypertension. J Thorac Dis. 2018; 10:508–21.

9. Gu S, Hu H, Dong H. Systematic review of health-related quality of life in patients with pulmonary arterial hypertension. Pharmacoeconomics. 2016; 34:751–70.

10. Ehlken N, Lichtblau M, Klose H, Weidenhammer J, Fischer C, Nechwatal R, et al. Exercise training improves peak oxygen consumption and haemodynamics in patients with severe pulmonary arterial hypertension and inoperable chronic thrombo-embolic pulmonary hypertension: a prospective, randomized, controlled trial. Eur Heart J. 2016; 37:35–44.

11. American College of Sports Medicine. ACSM’s resource manual for guideline for exercise testing and prescription. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins;2013.
12. Mereles D, Ehlken N, Kreuscher S, Ghofrani S, Hoeper MM, Halank M, et al. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation. 2006; 114:1482–9.

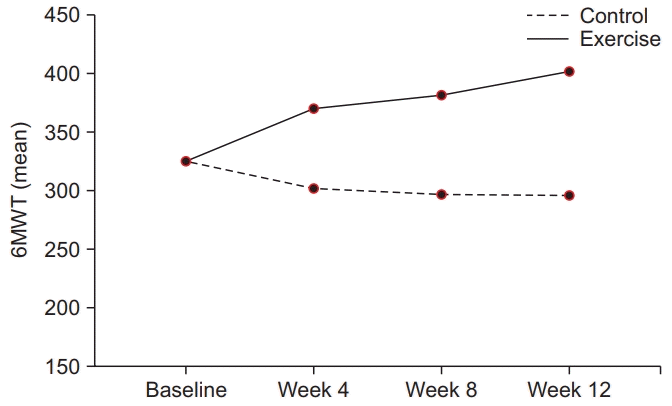
13. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002; 166:111–7.
14. Brooks R. EuroQol: the current state of play. Health Policy. 1996; 37:53–72.

15. Tongsiri S, Cairns J. Estimating population-based values for EQ-5D health states in Thailand. Value Health. 2011; 14:1142–5.

16. Endarti D, Riewpaiboon A, Thavorncharoensap M, Praditsitthikorn N, Hutubessy R, Kristina SA. A comparison of EQ-5D-3L index scores using Malaysian, Singaporean, Thai, and UK value sets in Indonesian cervical cancer patients. Value Health Reg Issues. 2018; 15:50–5.

17. Ehlken N, Verduyn C, Tiede H, Staehler G, Karger G, Nechwatal R, Opitz CF, et al. Economic evaluation of exercise training in patients with pulmonary hypertension. Lung. 2014; 192:359–66.

18. Fox BD, Kassirer M, Weiss I, Raviv Y, Peled N, Shitrit D, et al. Ambulatory rehabilitation improves exercise capacity in patients with pulmonary hypertension. J Card Fail. 2011; 17:196–200.

19. Richter MJ, Grimminger J, Kruger B, Ghofrani HA, Mooren FC, Gall H, et al. Effects of exercise training on pulmonary hemodynamics, functional capacity and inflammation in pulmonary hypertension. Pulm Circ. 2017; 7:20–37.

20. Amedro P, Guillaumont S, Bredy C, Matecki S, Gavotto A. Atrial septal defect and exercise capacity: value of cardio-pulmonary exercise test in assessment and follow-up. J Thorac Dis. 2018; 10(Suppl 24):S2864–73.

21. Nashat H, Montanaro C, Li W, Kempny A, Wort SJ, Dimopoulos K, et al. Atrial septal defects and pulmonary arterial hypertension. J Thorac Dis. 2018; 10(Suppl 24):S2953–65.

22. Marra AM, Arcopinto M, Bossone E, Ehlken N, Cittadini A, Grunig E. Pulmonary arterial hypertension-related myopathy: an overview of current data and future perspectives. Nutr Metab Cardiovasc Dis. 2015; 25:131–9.

23. Tran DL, Lau EMT, Celermajer DS, Davis GM, Cordina R. Pathophysiology of exercise intolerance in pulmonary arterial hypertension. Respirology. 2018; 23:148–59.

24. Dyer MT, Goldsmith KA, Sharples LS, Buxton MJ. A review of health utilities using the EQ-5D in studies of cardiovascular disease. Health Qual Life Outcomes. 2010; 8:13.

25. Pepke-Zaba J, Gilbert C, Collings L, Brown MC. Sildenafil improves health-related quality of life in patients with pulmonary arterial hypertension. Chest. 2008; 133:183–9.

26. Chan L, Chin LMK, Kennedy M, Woolstenhulme JG, Nathan SD, Weinstein AA, et al. Benefits of intensive treadmill exercise training on cardiorespiratory function and quality of life in patients with pulmonary hypertension. Chest. 2013; 143:333–43.

27. Kukkonen M, Puhakka A, Halme M. Quality of life among pulmonary hypertension patients in Finland. Eur Clin Respir J. 2016; 3:26405.

28. Grunig E, Lichtblau M, Ehlken N, Ghofrani HA, Reichenberger F, Staehler G, et al. Safety and efficacy of exercise training in various forms of pulmonary hypertension. Eur Respir J. 2012; 40:84–92.

29. Eshtehardi P, Mojadidi MK, Khosraviani K, Pamerla M, Zolty R. Effect of digoxin on mortality in patients with isolated right ventricular dysfunction secondary to severe pulmonary hypertension. J Am Coll Cardiol. 2014; 63(12_Suppl):A750.

30. So PP, Davies RA, Chandy G, Stewart D, Beanlands RS, Haddad H, et al. Usefulness of beta-blocker therapy and outcomes in patients with pulmonary arterial hypertension. Am J Cardiol. 2012; 109:1504–9.

31. van Campen JS, de Boer K, van de Veerdonk MC, van der Bruggen CE, Allaart CP, Raijmakers PG, et al. Bisoprolol in idiopathic pulmonary arterial hypertension: an explorative study. Eur Respir J. 2016; 48:787–96.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download