1. Garry EM, Fleetwood-Walker SM. A new view on how AMPA receptors and their interacting proteins mediate neuropathic pain. Pain. 2004; 109:210–3.

2. Kopach O, Voitenko N. Extrasynaptic AMPA receptors in the dorsal horn: evidence and functional significance. Brain Res Bull. 2013; 93:47–56.

3. Park JS, Voitenko N, Petralia RS, Guan X, Xu JT, Steinberg JP, et al. Persistent inflammation induces GluR2 internalization via NMDA receptor-triggered PKC activation in dorsal horn neurons. J Neurosci. 2009; 29:3206–19.

4. Kopach O, Kao SC, Petralia RS, Belan P, Tao YX, Voitenko N. Inflammation alters trafficking of extrasynaptic AMPA receptors in tonically firing lamina II neurons of the rat spinal dorsal horn. Pain. 2011; 152:912–23.

5. Tsuda M. Microglia in the spinal cord and neuropathic pain. J Diabetes Investig. 2016; 7:17–26.

6. Taves S, Berta T, Chen G, Ji RR. Microglia and spinal cord synaptic plasticity in persistent pain. Neural Plast. 2013; 2013:753656.

7. Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009; 10:23–36.

8. Mulleman D, Mammou S, Griffoul I, Watier H, Goupille P. Pathophysiology of disk-related sciatica. I.--Evidence supporting a chemical component. Joint Bone Spine. 2006; 73:151–8.

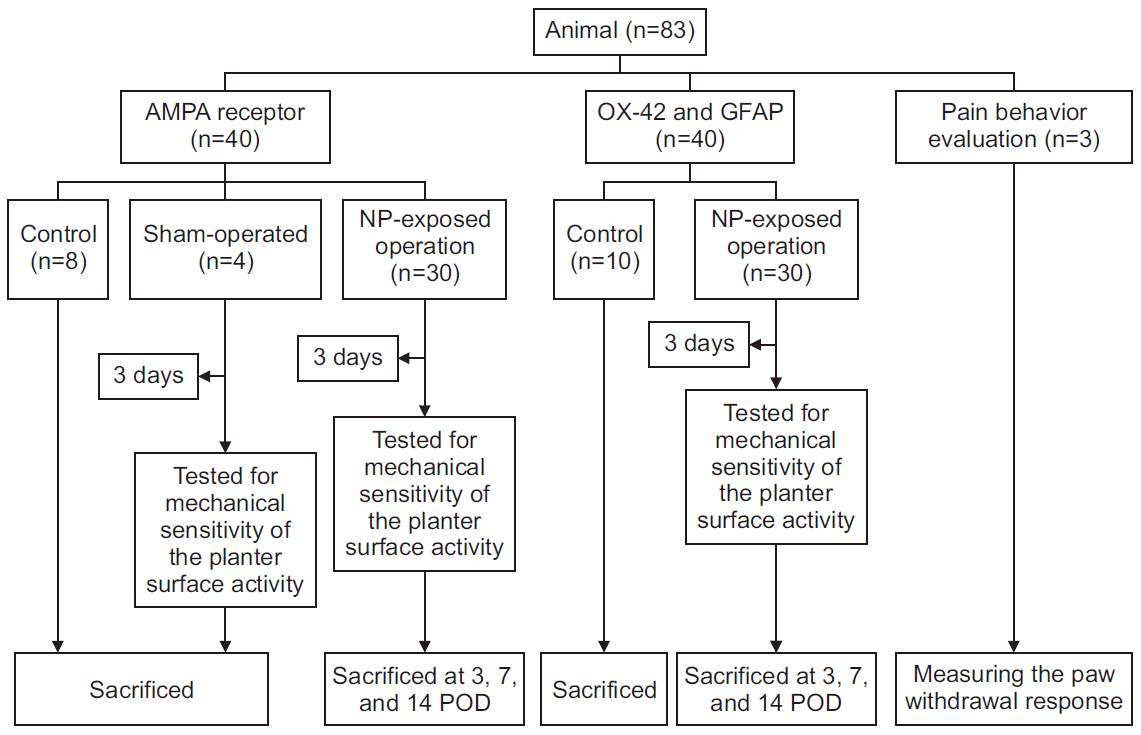
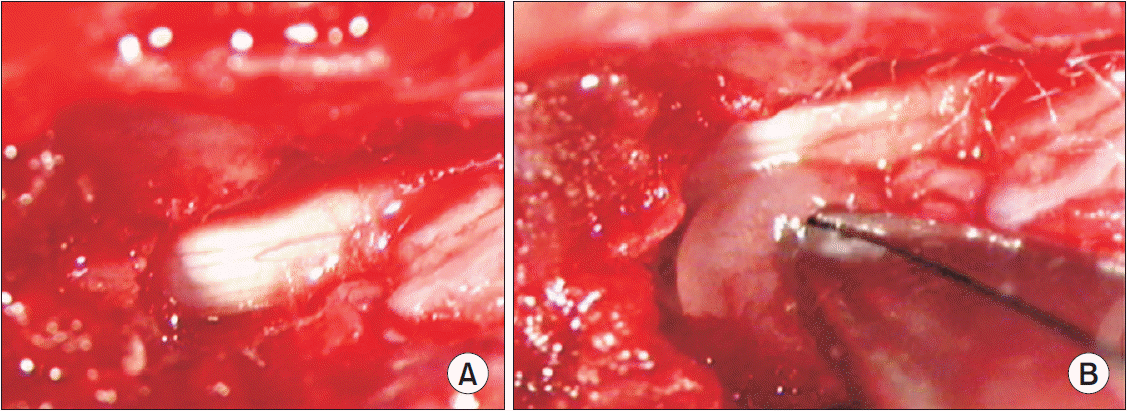
9. Sasaki N, Sekiguchi M, Shishido H, Kikuchi S, Yabuki S, Konno S. A comparison of pain-related behavior following local application of nucleus pulposus and/or mechanical compression on the dorsal root ganglion. Fukushima J Med Sci. 2011; 57:46–53.

10. Tachihara H, Sekiguchi M, Kikuchi S, Konno S. Do corticosteroids produce additional benefit in nerve root infiltration for lumbar disc herniation? Spine (Phila Pa 1976). 2008; 33:743–7.

11. Uesugi K, Sekiguchi M, Kikuchi S, Konno S. The effect of repeated restraint stress in pain-related behavior induced by nucleus pulposus applied on the nerve root in rats. Eur Spine J. 2011; 20:1885–91.

12. Park HW, Ahn SH, Son JY, Kim SJ, Hwang SJ, Cho YW, et al. Pulsed radiofrequency application reduced mechanical hypersensitivity and microglial expression in neuropathic pain model. Pain Med. 2012; 13:1227–34.

13. Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 2010; 16:1248–57.

14. Cho JH, Lee DG. Translocation of AMPA receptors in the dorsal horn of the spinal cord corresponding to long-term depression following pulsed radiofrequency stimulation at the dorsal root ganglion. Pain Med. 2019; pnz307.

15. Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009; 139:267–84.

16. Engelman HS, Allen TB, MacDermott AB. The distribution of neurons expressing calcium-permeable AMPA receptors in the superficial laminae of the spinal cord dorsal horn. J Neurosci. 1999; 19:2081–9.

17. Wigerblad G, Huie JR, Yin HZ, Leinders M, Pritchard RA, Koehrn FJ, et al. Inflammation-induced GluA1 trafficking and membrane insertion of Ca2+ permeable AMPA receptors in dorsal horn neurons is dependent on spinal tumor necrosis factor, PI3 kinase and protein kinase A. Exp Neurol. 2017; 293:144–58.
18. Kim KJ, Lee SH, Hwang SJ, Oh SJ. Expression of pERK in the neurons and microglia in the spinal cord of the rat with neuropathic pain. J Korean Neurotraumatol Soc. 2005; 1:19–29.

19. Buenaventura RM, Datta S, Abdi S, Smith HS. Systematic review of therapeutic lumbar transforaminal epidural steroid injections. Pain Physician. 2009; 12:233–51.
20. Gong K, Kung LH, Magni G, Bhargava A, Jasmin L. Increased response to glutamate in small diameter dorsal root ganglion neurons after sciatic nerve injury. PLoS One. 2014; 9:e95491.

21. Youn DH, Gerber G, Sather WA. Ionotropic glutamate receptors and voltage-gated Ca2+ channels in long-term potentiation of spinal dorsal horn synapses and pain hypersensitivity. Neural Plast. 2013; 2013:654257.
22. Leem JW, Kim HK, Hulsebosch CE, Gwak YS. Ionotropic glutamate receptors contribute to maintained neuronal hyperexcitability following spinal cord injury in rats. Exp Neurol. 2010; 224:321–4.

23. Verkhratsky A, Kirchhoff F. Glutamate-mediated neuronal-glial transmission. J Anat. 2007; 210:651–60.

24. Wang Y, Wu J, Wu Z, Lin Q, Yue Y, Fang L. Regulation of AMPA receptors in spinal nociception. Mol Pain. 2010; 6:5.

25. Matthias K, Kirchhoff F, Seifert G, Huttmann K, Matyash M, Kettenmann H, et al. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003; 23:1750–8.

26. Chen SR, Zhou HY, Byun HS, Pan HL. Nerve injury increases GluA2-lacking AMPA receptor prevalence in spinal cords: functional significance and signaling mechanisms. J Pharmacol Exp Ther. 2013; 347:765–72.

27. Park JS, Yaster M, Guan X, Xu JT, Shih MH, Guan Y, et al. Role of spinal cord alpha-amino-3-hydroxy5-methyl-4-isoxazolepropionic acid receptors in complete Freund’s adjuvant-induced inflammatory pain. Mol Pain. 2008; 4:67.

28. Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: a review. Anesth Analg. 2003; 97:1108–16.

29. Tao YX. Dorsal horn alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking in inflammatory pain. Anesthesiology. 2010; 112:1259–65.
30. Elward K, Gasque P. “Eat me” and “don’t eat me” signals govern the innate immune response and tissue repair in the CNS: emphasis on the critical role of the complement system. Mol Immunol. 2003; 40:85–94.

31. Lopez-Bayghen E, Espinoza-Rojo M, Ortega A. Glutamate down-regulates GLAST expression through AMPA receptors in Bergmann glial cells. Brain Res Mol Brain Res. 2003; 115:1–9.
32. Sivakumar V, Ling EA, Lu J, Kaur C. Role of glutamate and its receptors and insulin-like growth factors in hypoxia induced periventricular white matter injury. Glia. 2010; 58:507–23.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download