Abstract
Objective
To verify the pharyngeal width at rest as a measurement that could be used to assess changes in the degree of dysphagia over time in stroke patients.
Methods
In a cohort of stroke patients, we performed serial measurements of the pharyngeal width at the midpoints of the second (C2) and third (C3) cervical vertebral bodies using lateral neck X-rays while the patients were at rest. The JOSCYL width, a parameter named after the first initial of each developers’ surname and defined as the average value of the upper and lower pharyngeal widths, was used to formulate the JOSCYL scale, which was calculated as the JOSCYL width × 100/neck circumference. All patients also underwent serial videofluoroscopic swallowing studies (VFSSs). The Spearman correlation analysis was used to detect correlations between the serial VFSS results, JOSCYL widths, and JOSCYL scale values.
Globally, stroke is a major cause of potential life lost [1]. In addition to the increased mortality associated with stroke, up to 37%–45% of stroke patients experience dysphagia [2]. Swallowing is a complex sensorimotor process, and dysfunction in any of the involved muscles and cranial nerves can lead to malnutrition, dehydration, and in severe cases, mortality due to aspiration and consequent pneumonia [3]. Physicians must, therefore, monitor the current degree of dysphagia, objectively identify changes in this condition over time, and select a dietary plan compatible with these changes [4].
The videofluoroscopic swallowing study (VFSS), a primary clinical evaluation tool, is currently considered the gold-standard clinical assessment method for dysphagia [5]. However, the VFSS can cause discomfort for patients who have difficulty positioning themselves upright or are not able to follow verbal commands for the study [6]. These challenges highlight the need for additional means of observing changes in the level of dysphagia for stroke patients.
Correct swallowing action requires the pharynx to receive signals from a homotopic corticobulbar connection [7,8]. An injured corticobulbar connection or brainstem lesion after stroke would theoretically weaken the ability of the pharynx to constrict. In such cases, the pharyngeal width at rest might be wider than normal, which could place the patient at risk of aspiration. Our previous study showed that aspiration tended to occur in patients with increased pharyngeal widths following stroke with the optimal cutoff 17.8 mm of the JOSCYL width for predicting aspiration in the whole stroke group [9], and that similar trends also occurred in certain populations, with the optimal cutoff 20.0 mm of JOSCYL width in the elderly dysphagia group [10]. In another study, the patients with Parkinson’s disease exhibited larger pharyngeal areas compared to healthy adult peers, which were associated with penetration or aspiration during swallowing [11]. The JOSCYL width (named using the initials of the developers’ surnames), the average of the two pharyngeal widths in one person, was designed to simply represent the pharyngeal area. To correct for the possible effect each person’s neck size could have on pharyngeal widths, we devised the JOSCYL scale (JOSCYL width × 100/neck circumference).
We hypothesized that the pharyngeal muscle constriction might increase as the stroke patient progresses from the acute phase to the subacute phase, which might lead to a decrease in pharyngeal width, lowering aspiration tendency. In this study, we observed changes in the pharyngeal width over the time following stroke onset and determined whether this parameter could be applied as a simple and effective measure to evaluate whether dysphagia has been alleviated in a stroke patient.
Between January 1, 2017, and March 31, 2019, 783 patients with stroke underwent the VFSSs at the Department of Physical Medicine and Rehabilitation in the university hospital in a prospective collection of data. Included, were 45 patients with acute stroke who met the following inclusion criteria: (1) the first stroke in their lifetime, (2) a clinical stroke diagnosis confirmed by computed tomography (CT) or magnetic resonance imaging (MRI) of the brain, (3) an alert mental status, and (4) the first VFSS performed within 30 days after the stroke onset with follow-up testing over 30 days after the stroke onset. The following exclusion criteria were also applied: (1) a previous neck surgery, (2) a history of tracheostomy (3) oro-nasopharyngeal cancer, (4) oral dysphagia that limits VFSS, and (5) esophageal dysphagia confirmed by VFSS. The age, sex, height, weight, and neck circumference were recorded for all participants (Table 1).
All aspects of the study, including enrollment, clinical assessment, VFSS, and statistical analysis protocols, were approved by the local Institutional Review Board of Hallym University Sacred Heart Hospital (No. 2017-I040). Written informed consent was obtained from all participants. This clinical trial was registered with the Clinical Research Information Service (CRIS), a primary registry associated with the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (CRIS registration number: KCT0002352).
Three physicians performed the VFSSs (Fig. 1) after acquiring lateral neck X-rays. To reduce head and neck movement, one physician ensured that the participant’s head remained in a straight position during the lateral neck X-ray. Subsequently, the participant’s neck was rotated, and the bilateral mandible lines were aligned for fluoroscopy. Specifically, the perpendicular lines A and B originated from the posterior pharyngeal wall and were located at positions corresponding to the midpoints of C2 and C3, respectively (Fig. 2). Line A was positioned around the lower margin of the mandible, while line B was positioned around the epiglottis. We used the default measurement program to determine the lengths (mm) of lines A and B on the screen directly connected with the fluoroscopy device. The average of the two pharyngeal widths ([A+B]/2) was designated the JOSCYL width. We then measured the neck circumference and calculated the JOSCYL scale.
Subsequently, each participant underwent a swallowing study. To stabilize their anatomical position, each participant initially sat laterally and was observed for 4–5 seconds. Three types of materials with different viscosities (25 mm, 55 mm, and 10 mm for semisolid, water, and solid, respectively) were tested [12]. A radiopaque material (barium sulfate, 960 mg/g; Solotop HD, Taejoon Pharm Co., Seoul, Korea) was administered with incremental increases in bolus volume and texture according to the participant’s tolerance [13,14]. For the semisolid test, barium was mixed with yogurt to a concentration of 40% w/v, and two tests were conducted with 2 mL and 5 mL volumes. For the water test, barium was diluted in water to yield a concentration of 20% w/v, and three tests were conducted with volumes of 2, 5, and 15 mL [15,16]. During the third step of the water test, the participants were allowed to self-administer water from a cup. For the solid test, a liquid thickener (Yeon-ha New EG; Blue Bio S, Gwangju, Korea) was mixed with water in a 1:1 ratio and formed into a ball with a diameter of approximately 13 mm. The solid test was performed once [17] and was not performed if the participant was unable to eat a solid diet because of dental problems. Three physicians who were blinded to the JOSCYL width data determined the Penetration-Aspiration Scale (PAS) [18] and the Dysphagia Outcome and Severity Scale (DOSS) scores [19] while watching recorded videos of the VFSS. The physicians chose the worst score of each participant’s PAS and DOSS scores regardless of the type of material used. Each participant also underwent a second test according to the above process at a later point.
We used G*Power Analysis (Version 3.1.9.2) to obtain the minimum number of the sample size required to achieve statistical significance and found that at least 34 patients were needed to have a power of 80% at a significance level of 0.05. We derived the JOSCYL widths and PAS and DOSS scores from all the patients in our study group who underwent multiple VFSSs. We then used a paired t-test to compare the first and serial JOSCYL width (A, A’), JOSCYL scale (B, B’), PAS (C, C’), and DOSS (D, D’) values of patients within this group. Differences between the first and serial values in each category were then calculated for each patient (ΔA=A’–A, ΔB=B’–B, ΔC=C’–C, ΔD=D’–D). Finally, the correlations of ΔA and ΔB with ΔC and ΔD were analyzed using a Spearman correlation analysis. Statistical significance was defined as a p-value of <0.05 and a confidence interval (CI) of >95% using SPSS version 24.0 (IBM Corp., Armonk, NY, USA).
The participants’ demographic data are shown in Table 1. Forty-five patients included 31 men and 14 women, with an average age of 70.2±12.7 years. Twenty-five and 20 patients had ischemic and hemorrhagic strokes, respectively. Regarding lesion location, 25, 3, and 6 patients had cerebral, cerebellar, and brainstem lesions, respectively. Eleven patients had a subarachnoid hemorrhage.
The first and second assessments were performed at an average of 16.9±6.4 and 110.7±175.9 days post-stroke, respectively. The follow-up JOSCYL width and scale values were significantly lower than the respective initial values (p<0.05 for both) (Table 2). Similarly, the followup PAS scores were lower than the initial scores (p<0.01). Conversely, the follow-up DOSS scores were significantly higher than the initial scores (p<0.05). Significant positive linear correlations were observed between changes in the PAS scores and changes in the JOSCYL widths and scales over time (r=0.563, p<0.01 and r=0.571, p<0.01, respectively), while significant negative linear correlations were observed between changes in the DOSS scores and changes in the JOSCYL widths and scales over time (r=-0.510, p<0.01 and r=-0.507, p<0.01, respectively) (Table 3, Fig. 3).
A large number of patients with acute stroke experience difficulty swallowing. The standard practice for the management of patients with dysphagia includes obtaining comprehensive clinical and instrumental evaluations. Although the VFSS is considered among the most reliable tests for confirming the degree of dysphagia [20,21], this test may cause discomfort to patients who have difficulty achieving a specific posture for it. Accordingly, some previous studies used simple lateral neck X-rays as an alternative method of dysphagia evaluation. Kendall and Leonard [22] observed that patients with dysphagia had a larger pharyngeal area at maximal contraction, which suggested poor pharyngeal contraction and was associated with an increased number of aspiration cases. Stokely et al. [23] reported a correlation of an increased pharyngeal area at maximal contraction with increased post-swallowing residue in the valleculae and pyriform sinuses. Molfenter et al. [24] found that increasing pharyngeal lumen volume by pharyngeal muscle atrophy in aging was significantly related to worse pharyngeal constriction and vallecular residue. In other words, those studies attribute aspiration to an increased pharyngeal area caused by pharyngeal muscle weakness. Accordingly, we measured the JOSCYL width, the pharyngeal width at rest, and found previously that both the absolute JOSCYL width and scale were valuable indicators of the risk of aspiration after stroke.
The premise of our previous articles was that if stroke lesions could injure corticobulbar connections, the muscle tone of the pharyngeal constrictors might decrease, and the pharyngeal width may increase [9]. In the present study, we aimed to use changes in the JOSCYL width and scale over time to confirm they have value as an easy method for monitoring changes in dysphagia in stroke patients. To achieve this aim, we hypothesized that the pharyngeal width would decrease as the muscle tone recovered over time. Notably, our results revealed that the change in the JOSCYL width over time exhibited positive and negative correlations with the changes in the PAS scores and DOSS scores over time, respectively. In other words, serial observations in the JOSCYL width over time may provide a simple follow-up method that will enable clinicians to select an appropriate diet for the stroke patient. In addition, clinicians can use these changes to plan to apply functional electrical stimulation to patients’ necks in order to recover from decreased muscle tone.
Checking the changes in JOSCYL width and scale over time would not be sufficient on their own for clinical decision-making regarding the next steps in dysphagia management for stroke patients. Specific diet recommendations, therapeutic exercises, and compensatory strategies must be based on a careful physiological assessment; however, it is important to note that the JOSCYL values may be one of several significant indicators that can assess the recovery of swallowing disorders.
There are no specified rules regarding the interval between the two VFSSs in this study because the first VFSSs included patients who were admitted to the acute stroke unit in the university hospital where our researchers belonged, and the second VFSSs usually included subacute or chronic stroke patients who were referred from other hospitals. Although the time between the two tests could not be adjusted uniformly, this study did not examine the change of swallowing according to the intervals between the two VFSSs, but rather the relation of the changes in pharyngeal width to the changes in swallowing between each test.
This study had some limitations. First, this study was limited by the patient heterogeneity in terms of the lesion sites, as well as the patient sample containing cases with corticobulbar connection problems, direct injuries to cranial nuclei related to swallowing function, and so on. If these cases are studied separately, results may differ, so further research is needed. Second, all participants were of Asian ethnicity. Therefore, it is unclear whether our results can be generalized for other ethnic groups. Third, the dysphagia therapies between the first VFSS and second VFSS of each patient could not be controlled since the second VFSSs included patients from various hospitals. Different dysphagia therapies could have influenced the results of the second VFSSs. Finally, the VFSS had to be discontinued at different levels of the testing for some patients due to the risk of aspiration pneumonia; therefore, it was impossible to obtain each PAS and DOSS value for the different dietary materials. The PAS and DOSS scores used in this study were the worst scores out of each participant’s PAS and DOSS scores regardless of the type of material. Further studies involving larger samples, homogenic stroke profile, multi-ethnic populations, and controlled dysphagia therapy between VFSSs are needed to determine whether changes in the JOSCYL width over time are truly reflective of the dysphagic condition.
In conclusion, we have demonstrated that changes in the JOSCYL width over time correlated positively and negatively with changes in the PAS and DOSS scores, respectively. Our findings suggest that the JOSCYL width and scale may be useful tools for evaluating serial changes in the dysphagic conditions of stroke patients, regardless of the duration of disease, and it may be a significant step towards beginning to make diet recommendations identifying the current level of swallowing without complex instrumental exams.
ACKNOWLEDGMENTS
The authors would like to thank Yun Jae Lee, Sun Woo Park, Wan Ho Noh, and Chan Sun Jung of the Department of Radiology, Hallym University Sacred Heart Hospital, for acquiring VFSS measurements.
REFERENCES
1. Kim AS, Cahill E, Cheng NT. Global stroke belt: geographic variation in stroke burden worldwide. Stroke. 2015; 46:3564–70.
2. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005; 36:2756–63.
3. Papadopoulou SL, Ploumis A, Exarchakos G, Theodorou SJ, Beris A, Fotopoulos AD. Versatility of repetitive transcranial magnetic stimulation in the treatment of poststroke dysphagia. J Neurosci Rural Pract. 2018; 9:391–6.

4. Smithard DG, O’Neill PA, Parks C, Morris J. Complications and outcome after acute stroke. Does dysphagia matter? Stroke. 1996; 27:1200–4.
5. Lan Y, Xu G, Dou Z, Lin T, Yu F, Jiang L. The correlation between manometric and videofluoroscopic measurements of the swallowing function in brainstem stroke patients with dysphagia. J Clin Gastroenterol. 2015; 49:24–30.

6. Gomes GF, Campos AC, Pisani JC, Macedo ED, Vieira MC. Diagnostic methods for the detection of anterograde aspiration in enterally fed patients. Curr Opin Clin Nutr Metab Care. 2004; 7:285–92.

7. Dodds WJ. Physiology of swallowing. Dysphagia. 1989; 3:171–8.
8. Mu L, Sanders I. Neuromuscular specializations within human pharyngeal constrictor muscles. Ann Otol Rhinol Laryngol. 2007; 116:604–17.

9. Jung IH, Lee HY, Cha E, Song J, Baek S, Jung KI, et al. Pharyngeal width and aspiration after stroke. Int J Rehabil Res. 2019; 42:152–9.

10. Lee HY, Jung IH, Cha E, Song J, Jung KI, Yoo WK, et al. Predictive value of pharyngeal width at rest (JOSCYL Width) for aspiration in elderly people. Ann Rehabil Med. 2019; 43:187–94.

11. Curtis JA, Molfenter SM, Troche MS. Pharyngeal area changes in Parkinson’s disease and its effect on swallowing safety, efficiency, and kinematics. Dysphagia. 2020; 35:389–98.

12. Nicosia MA, Robbins J. The usefulness of the line spread test as a measure of liquid consistency. Dysphagia. 2007; 22:306–11.

13. Edmiaston J, Connor LT, Loehr L, Nassief A. Validation of a dysphagia screening tool in acute stroke patients. Am J Crit Care. 2010; 19:357–64.

14. Han TR, Paik NJ, Park JW. Quantifying swallowing function after stroke: a functional dysphagia scale based on videofluoroscopic studies. Arch Phys Med Rehabil. 2001; 82:677–82.

15. Eisenhuber E, Schima W, Schober E, Pokieser P, Stadler A, Scharitzer M, et al. Videofluoroscopic assessment of patients with dysphagia: pharyngeal retention is a predictive factor for aspiration. AJR Am J Roentgenol. 2002; 178:393–8.
16. Jaffer NM, Ng E, Au FW, Steele CM. Fluoroscopic evaluation of oropharyngeal dysphagia: anatomic, technical, and common etiologic factors. AJR Am J Roentgenol. 2015; 204:49–58.

18. Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996; 11:93–8.

19. O’Neil KH, Purdy M, Falk J, Gallo L. The dysphagia outcome and severity scale. Dysphagia. 1999; 14:139–45.

20. Horiguchi S, Suzuki Y. Screening tests in evaluating swallowing function. Japan Med Assoc J. 2011; 54:31–4.
21. Leigh JH, Lim JY, Han MK, Bae HJ, Kim WS, Paik NJ. A prospective comparison between bedside swallowing screening test and videofluoroscopic swallowing study in post-stroke dysphagia. Brain Neurorehabil. 2016; Sep. 9(2):e7.

22. Kendall KA, Leonard RJ. Pharyngeal constriction in elderly dysphagic patients compared with young and elderly nondysphagic controls. Dysphagia. 2001; 16:272–8.

Fig. 1.
Flow chart of the analytical process. Lateral cervical spine X-rays were taken prior to the videofluoroscopic swallowing study (VFSS), which used three types of materials with different viscosities. PAS, Penetration-Aspiration Scale; DOSS, Dysphagia Outcome and Severity Scale.
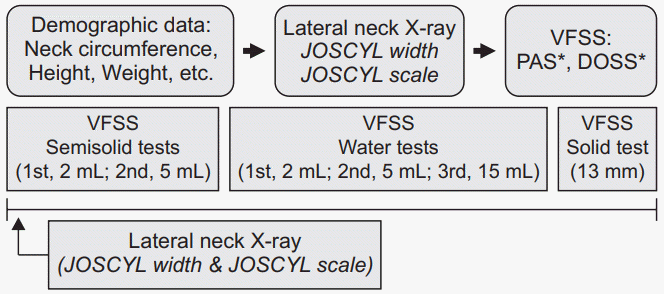
Fig. 2.
Determination of the JOSCYL width on the cervical X-ray. This value was calculated as the average of two pharyngeal widths, A and B. The perpendicular lines A and B originated from the posterior pharyngeal wall at the positions corresponding to the midpoints of the second and third cervical vertebral bodies, respectively. Lines A and B were positioned around the lower margin of the mandible and around the epiglottis, respectively.
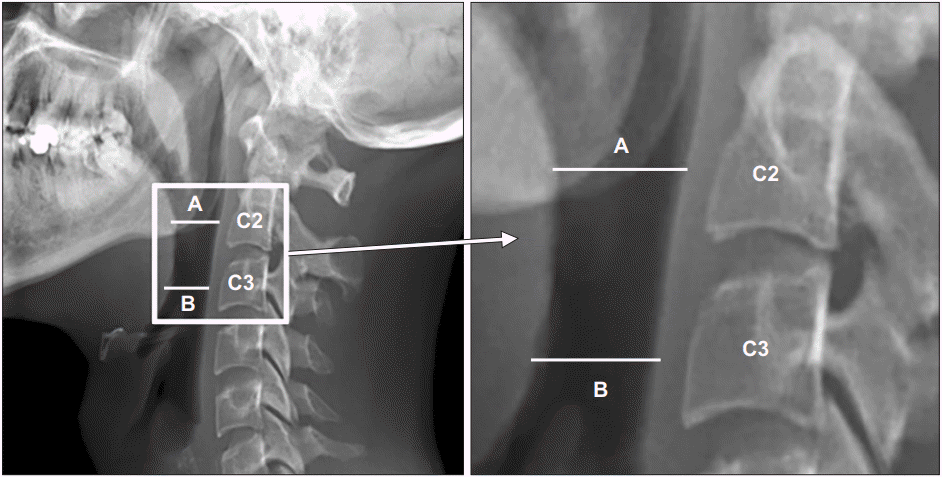
Fig. 3.
Analysis of correlations between changes in the JOSCYL width (A) and JOSCYL scale (B) over time and changes in the dysphagia scale scores over time. The JOSCYL width and scale correlated positively with the Penetration-Aspiration Scale (PAS) scores and negatively with the Dysphagia Outcome and Severity Scale (DOSS) scores over time. All correlations were significant.
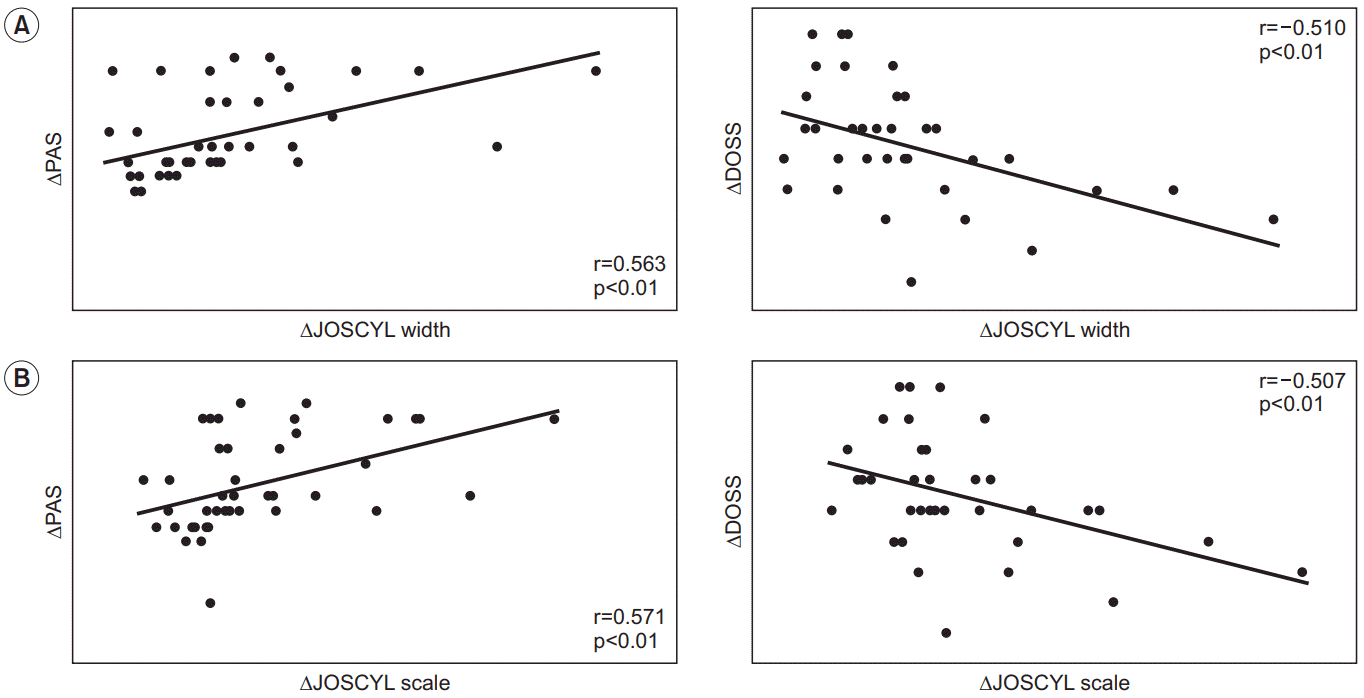
Table 1.
Clinical characteristics of the participants (n=45)
Table 2.
Changes in JOSCYL and swallowing scale values over time
| 1st VFSS | 2nd VFSS | p-value | |
|---|---|---|---|
| JOSCYL width (mm) | 18.23±0.90 | 16.85±0.90 | <0.05 |
| JOSCYL scale | 5.1±1.6 | 4.7±1.6 | <0.05 |
| PAS | 6.20±2.26 | 4.49±2.79 | <0.01 |
| DOSS | 3.64±1.32 | 4.18±1.40 | <0.05 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download