INTRODUCTION
MATERIALS AND METHODS
Subjects
Outcome measurements
Statistical analysis
RESULTS
Basic characteristics
Table 1.
|
All stroke |
Ischemic stroke |
Hemorrhagic stroke |
p-valueb) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aphasia (n=67) | Control (n=46) | p-valuea) | Aphasia (n=39) | Control (n=37) | p-valuea) | Aphasia (n=28) | Control (n=9) | p-valuea) | ||
| Age (yr) | 68.87±11.87 | 66.24±12.72 | 0.122 | 72.44±11.33 | 68.49±12.27 | 0.056 | 63.89±10.94 | 57.00±10.67 | 0.073 | 0.002* |
| Male (%) | 33 (49.25) | 29 (63.04) | 0.180 | 25 (64.10) | 25 (67.57) | 0.812 | 8 (28.57) | 4 (44.44) | 0.432 | 0.006* |
| Lt. hemisphere (%) | 35 (52.24) | 19 (41.30) | 0.118 | 25 (64.10) | 14 (38.46) | 0.038* | 10 (47.62) | 5 (55.56) | 0.234 | 0.276 |
| DM (%) | 16 (23.88) | 20 (43.48) | 0.040* | 12 (30.77) | 13 (35.14) | 0.808 | 4 (14.29) | 7 (77.78) | 0.001* | 0.152 |
| Hypertension (%) | 39 (58.21) | 30 (65.22) | 0.556 | 24 (61.54) | 23 (62.16) | 1.000 | 15 (53.57) | 7 (77.78) | 0.262 | 0.618 |
| Tracheostomy (%) | 10 (14.93) | 0 (0.0) | 0.005* | 1 (2.56) | 0 (0) | 1.000 | 9 (32.14) | 0 (0) | 0.079 | 0.001* |
| Pneumonia (%) | 16 (23.88) | 2 (4.35) | 0.007* | 6 (15.38) | 2 (5.41) | 0.265 | 10 (35.71) | 0 (0) | 0.079 | 0.081 |
| Dysphagia | 46 (68.66) | 17 (36.96) | 0.001* | 29 (74.36) | 17 (45.95) | 0.018* | 17 (60.71) | 0 (0) | 0.002* | 0.290 |
| PAS | 3.90±2.90 | 4.35±2.89 | 0.707 | 3.89±2.87 | 4.50±2.87 | 0.548 | 3.91±2.99 | 1.00±0 | 0.261 | 0.936 |
| Depression (%) | 12 (17.91) | 3 (6.52) | 0.099 | 8 (20.51) | 1 (2.70) | 0.029* | 4 (14.29) | 2 (22.22) | 0.620 | 0.748 |
| Initial NIHSS | 11.91±5.98 | 4.80±3.43 | 0.000* | 3.89±2.87 | 4.50±2.87 | 0.000* | 12.68±6.33 | 7.25±3.49 | 0.031* | 0.333 |
Values are presented as mean±standard deviation or number (%).
DM, diabetes mellitus; PAS, Penetration Aspiration Scale; NIHSS, National Institutes of Health Stroke Scale.
a) Mann-Whitney U-test or Fisher exact test compared between aphasic patients and non-aphasic controls.
Types and severity of aphasia
Table 2.
| Ischemic (n=39) | Hemorrhagic (n=28) | p-value | |
|---|---|---|---|
| Aphasia types | |||
| Global | 19 (48.72) | 8 (28.57) | 0.024* |
| Broca’s | 1 (2.56) | 5 (17.86) | |
| Wernicke’s | 3 (7.69) | 7 (25.00) | |
| Transcortical motor | 0 (0) | 0 (0) | |
| Transcortical sensory | 0 (0) | 2 (7.14) | |
| Transcortical mixed | 1 (2.56) | 0 (0) | |
| Conduction | 1 (2.56) | 0 (0) | |
| Anomic | 14 (35.90) | 6 (21.43) | |
| K-FAST | 6.64±7.81 | 4.21±4.49 | 0.387 |
| K-WAB | |||
| Spontaneous speech | 7.56±6.08 | 6.64±5.44 | 0.408 |
| Comprehension | 87.21±72.88 | 87.25±65.35 | 0.990 |
| Repetition | 38.72±40.68 | 40.68±38.21 | 0.918 |
| Naming | 40.82±37.73 | 32.75±33.97 | 0.278 |
| Aphasia quotient | 40.79±40.30 | 36.63±29.16 | 0.576 |
Cognitive and functional outcomes
Table 3.
| K-MMSE |
All stroke |
Ischemic stroke |
Hemorrhagic stroke |
p-valueb) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aphasia (n=67) | Control (n=46) | p-valuea) | Aphasia (n=39) | Control (n=37) | p-valuea) | Aphasia (n=28) | Control (n=9) | p-valuea) | |||
| Orientation | |||||||||||
| Initial | 2.72±3.30 | 8.40±1.84 | 0.000* | 3.21±3.72 | 8.83±1.44 | 0.000* | 2.04±2.52 | 6.50±2.27 | 0.001* | 0.299 | |
| Follow-up | 4.02±3.74 | 9.31±1.09 | 0.000* | 4.21±4.11 | 9.47±0.93 | 0.000* | 3.75±3.22 | 8.63±1.51 | 0.000* | 0.672 | |
| Gain | 1.32±2.54 | 1.00±1.75 | 0.772 | 1.03±2.62 | 0.77±1.68 | 0.816 | 1.71±2.43 | 1.89±1.83 | 0.636 | 0.299 | |
| p-valuec) | 0.000* | 0.000* | 0.014* | 0.010* | 0.002* | 0.017* | |||||
| Attention | |||||||||||
| Initial | 0.60±1.32 | 2.79±1.75 | 0.000* | 0.79±1.49 | 3.11±1.71 | 0.000* | 0.32±0.98 | 1.38±1.19 | 0.002* | 0.137 | |
| Follow-up | 0.93±1.50 | 3.29±1.73 | 0.000* | 1.08±1.72 | 3.44±1.78 | 0.000* | 0.71±1.12 | 2.63±1.41 | 0.002* | 0.738 | |
| Gain | 0.33±1.28 | 0.48±1.44 | 0.107 | 0.28±1.36 | 0.31±1.39 | 0.120 | 0.39±1.20 | 1.11±1.54 | 0.235 | 0.165 | |
| p-valuec) | 0.049* | 0.013* | 0.332 | 0.072 | 0.070 | 0.068 | |||||
| Recall | |||||||||||
| Initial | 0.46±0.91 | 1.58±1.22 | 0.000* | 0.56±1.05 | 1.66±1.21 | 0.000* | 0.32±0.67 | 1.25±1.28 | 0.018* | 0.518 | |
| Follow-up | 0.78±1.11 | 2.17±1.10 | 0.000* | 0.77±1.11 | 2.32±1.00 | 0.000* | 0.79±1.13 | 1.50±1.31 | 0.165 | 0.948 | |
| Gain | 0.31±0.94 | 0.55±1.07 | 0.544 | 0.21±0.95 | 0.63±1.06 | 0.194 | 0.46±0.92 | 0.22±1.09 | 0.366 | 0.402 | |
| p-valuec) | 0.009* | 0.003* | 0.186 | 0.004* | 0.015* | 0.458 | |||||
| Language | |||||||||||
| Initial | 2.73±3.22 | 7.60±1.64 | 0.000* | 3.13±3.37 | 7.86±1.61 | 0.000* | 2.18±2.96 | 6.50±1.31 | 0.001* | 0.238 | |
| Follow-up | 4.03±3.39 | 8.025±1.60 | 0.000* | 3.85±3.55 | 8.15±1.60 | 0.000* | 4.29±3.20 | 7.50±1.60 | 0.009* | 0.764 | |
| Gain | 1.30±2.75 | 0.52±1.42 | 0.216 | 0.72±2.77 | 0.43±1.52 | 0.706 | 2.11±2.56 | 0.89±0.93 | 0.451 | 0.050 | |
| p-valuec) | 0.000* | 0.013* | 0.067 | 0.103 | 0.001* | 0.038* | |||||
| Total | |||||||||||
| Initial | 7.82±8.91 | 23.28±5.07 | 0.000* | 8.95±9.81 | 24.22±4.49 | 0.000* | 6.25±7.36 | 19.44±5.79 | 0.000* | 0.337 | |
| Follow-up | 11.54±10.02 | 25.78±4.11 | 0.000* | 11.54±10.88 | 26.27±3.90 | 0.000* | 11.54±8.88 | 23.78±4.60 | 0.000* | 0.871 | |
| Gain | 3.76±6.52 | 2.50±3.04 | 0.935 | 2.63±6.59 | 2.05±2.89 | 0.686 | 5.29±6.23 | 4.33±3.12 | 1.000 | 0.108 | |
| p-valuec) | 0.000* | 0.000* | 0.009* | 0.000* | 0.000* | 0.018* | |||||
Table 4.
| K-MBI |
All stroke |
Ischemic stroke |
Hemorrhagic stroke |
p-valueb) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aphasia (n=67) | Control (n=46) | p-valuea) | Aphasia (n=39) | Control (n=37) | p-valuea) | Aphasia (n=28) | Control (n=9) | p-valuea) | |||
| Self-care | |||||||||||
| Initial | 13.91±16.27 | 38.02±16.27 | 0.000* | 18.90±18.42 | 38.62±16.52 | 0.000* | 6.96±10.46 | 35.56±15.88 | 0.000* | 0.003* | |
| Follow-up | 28.63±21.56 | 53.88±13.88 | 0.000* | 34.87±21.89 | 53.50±13.86 | 0.000* | 19.93±18.07 | 55.50±14.82 | 0.000* | 0.006* | |
| Gain | 14.72±16.12 | 11.17±19.89 | 0.736 | 13.45±12.56 | 15.33±15.79 | 0.617 | 12.96±15.49 | 17.10±25.24 | 0.188 | 0.455 | |
| p-valuec) | 0.000* | 0.000* | 0.000* | 0.000* | 0.000* | 0.012* | |||||
| Mobility | |||||||||||
| Initial | 4.78±7.71 | 12.33±8.63 | 0.000* | 7.10±9.25 | 13.05±8.82 | 0.001* | 1.54±2.50 | 9.33±7.52 | 0.001* | 0.002* | |
| Follow-up | 10.57±10.01 | 21.69±7.28 | 0.000* | 13.08±10.76 | 21.44±6.88 | 0.000* | 7.07±7.77 | 22.75±9.24 | 0.000* | 0.015* | |
| Gain | 5.79±7.13 | 7.48±9.77 | 0.195 | 7.27±8.15 | 6.10±8.15 | 0.688 | 5.54±7.25 | 11.40±10.22 | 0.040* | 0.721 | |
| p-valuec) | 0.000* | 0.000* | 0.000* | 0.000* | 0.000* | 0.012* | |||||
| Total | |||||||||||
| Initial | 18.69±22.82 | 50.34±23.52 | 0.000* | 26.00±25.84 | 51.68±23.78 | 0.000* | 8.50±12.19 | 44.89±22.93 | 0.000* | 0.002* | |
| Follow-up | 39.19±30.63 | 75.57±19.65 | 0.000* | 47.95±31.58 | 74.86±19.36 | 0.000* | 27.00±25.00 | 78.44±21.76 | 0.000* | 0.006* | |
| Gain | 20.51±21.36 | 25.22±17.60 | 0.060 | 21.95±21.30 | 23.19±17.86 | 0.444 | 18.50±21.68 | 36.50±16.51 | 0.023* | 0.466 | |
| p-valuec) | 0.000* | 0.000* | 0.000* | 0.000* | 0.000* | 0.008* | |||||
Relationship between aphasia severity and cognitive and functional outcomes
Table 5.
|
All stroke |
Ischemic stroke |
Hemorrhagic stroke |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | Com | Rep | Nam | AQ | SS | Com | Rep | Nam | AQ | SS | Com | Rep | Nam | AQ | ||
| K-MMSE | ||||||||||||||||
| Initial | 0.753* | 0.771* | 0.742* | 0.771* | 0.807* | 0.795* | 0.861* | 0.779* | 0.848* | 0.849* | 0.656* | 0.597* | 0.662* | 0.602* | 0.682* | |
| Follow-up | 0.652* | 0.680* | 0.702* | 0.726* | 0.707* | 0.726* | 0.749* | 0.765* | 0.815* | 0.761* | 0.465* | 0.540* | 0.592* | 0.524* | 0.549* | |
| Gain | 0.054 | -0.004 | 0.207 | 0.080 | 0.059 | 0.054 | -0.014 | 0.215 | 0.084 | 0.040 | 0.055 | 0.038 | 0.199 | 0.107 | 0.076 | |
| K-MBI | ||||||||||||||||
| Initial | 0.560* | 0.525* | 0.436* | 0.526* | 0.530* | 0.575* | 0.584* | 0.467* | 0.579* | 0.551* | 0.507* | 0.542* | 0.426* | 0.444* | 0.523* | |
| Follow-up | 0.368* | 0.343* | 0.339* | 0.374* | 0.324* | 0.407* | 0.458* | 0.424* | 0.466* | 0.380* | 0.252 | 0.230* | 0.261 | 0.114 | 0.231 | |
| Gain | 0.042 | 0.002 | 0.126 | 0.057 | -0.001 | 0.053 | 0.035 | 0.167 | 0.112 | 0.004 | 0.009 | -0.034 | 0.083 | -0.126 | -0.020 | |
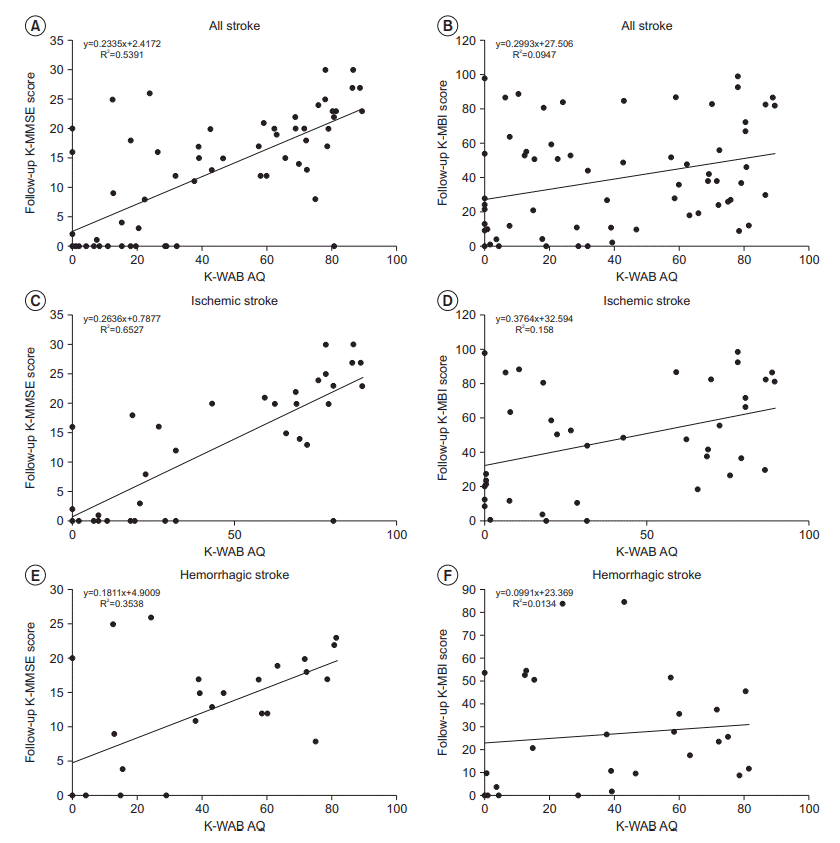 | Fig. 1.Regression analysis of (A, C, E) K-MMSE and (B, D, F) K-MBI total scores in all stroke patients with aphasia (A, B), ischemic stroke patients with aphasia (C, D), and hemorrhagic stroke patients with aphasia (E, F) at follow-up according to K-WAB Aphasia Quotient (AQ) at admission. The solid lines indicate regression lines. K-MMSE, Korean version of Mini-Mental State Examination; K-MBI, Korean version of Modified Barthel Index; K-WAB, Korean version of Western Aphasia Battery. |
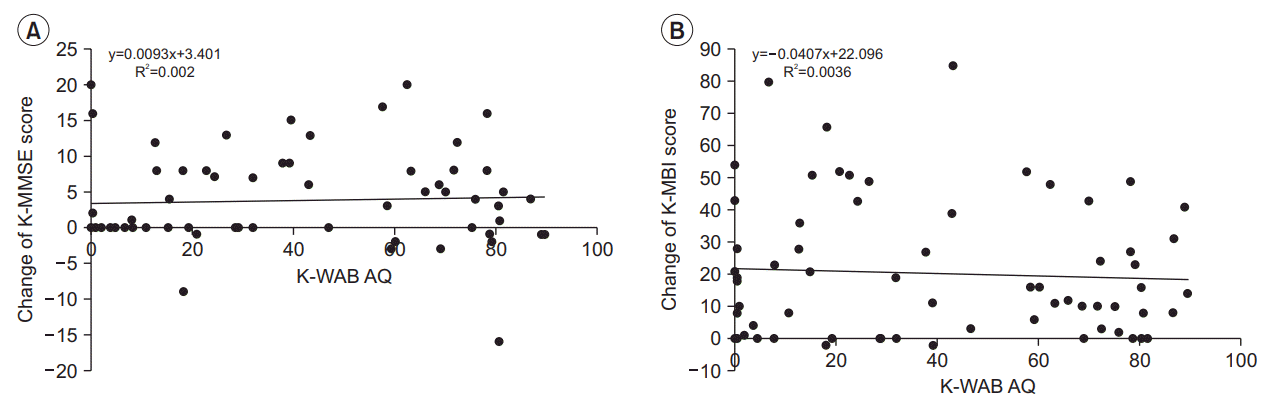 | Fig. 2.Regression analysis of (A) K-MMSE, and (B) K-MBI gain scores during follow-up period according to K-WAB Aphasia Quotient (AQ) at admission. The solid lines indicate regression lines. K-MMSE, Korean version of Mini-Mental State Examination; K-MBI, Korean version of Modified Barthel Index; K-WAB, Korean version of Western Aphasia Battery. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download